Does Direct and Indirect Exposure to Ionising Radiation Influence the Metastatic Potential of Breast Cancer Cells
- PMID: 31963587
- PMCID: PMC7016586
- DOI: 10.3390/cancers12010236
Does Direct and Indirect Exposure to Ionising Radiation Influence the Metastatic Potential of Breast Cancer Cells
Abstract
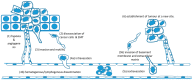
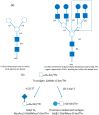
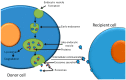
Ionising radiation (IR) is commonly used for cancer therapy; however, its potential influence on the metastatic ability of surviving cancer cells exposed directly or indirectly to IR remains controversial. Metastasis is a multistep process by which the cancer cells dissociate from the initial site, invade, travel through the blood stream or lymphatic system, and colonise distant sites. This complex process has been reported to require cancer cells to undergo epithelial-mesenchymal transition (EMT) by which the cancer cells convert from an adhesive, epithelial to motile, mesenchymal form and is also associated with changes in glycosylation of cell surface proteins, which may be functionally involved in metastasis. In this paper, we give an overview of metastatic mechanisms and of the fundamentals of cancer-associated glycosylation changes. While not attempting a comprehensive review of this wide and fast moving field, we highlight some of the accumulating evidence from in vitro and in vivo models for increased metastatic potential in cancer cells that survive IR, focusing on angiogenesis, cancer cell motility, invasion, and EMT and glycosylation. We also explore the indirect effects in cells exposed to exosomes released from irradiated cells. The results of such studies need to be interpreted with caution and there remains limited evidence that radiotherapy enhances the metastatic capacity of cancers in a clinical setting and undoubtedly has a very positive clinical benefit. However, there is potential that this therapeutic benefit may ultimately be enhanced through a better understanding of the direct and indirect effects of IR on cancer cell behaviour.
Keywords: EMT; epithelial mesenchymal transition; exosomes; glycosylation; invasion; ionising radiation; metastasis.
Conflict of interest statement
We would like to confirm that there is no conflict of interest.
Figures
Similar articles
-
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.Mol Cancer. 2017 Jan 30;16(1):10. doi: 10.1186/s12943-016-0577-4. Mol Cancer. 2017. PMID: 28137309 Free PMC article. Review.
-
Therapeutic Fractional Doses of Ionizing Radiation Promote Epithelial-Mesenchymal Transition, Enhanced Invasiveness, and Altered Glycosylation in MCF-7 Breast Cancer Cells.Genome Integr. 2023 May 4;14:e20230001. doi: 10.14293/genint.14.1.002. eCollection 2023. Genome Integr. 2023. PMID: 38025522 Free PMC article.
-
Molecular alterations that drive breast cancer metastasis to bone.Bonekey Rep. 2015 Mar 18;4:643. doi: 10.1038/bonekey.2015.10. eCollection 2015. Bonekey Rep. 2015. PMID: 25848532 Free PMC article. Review.
-
Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression.Semin Cell Dev Biol. 2015 Apr;40:60-71. doi: 10.1016/j.semcdb.2015.02.008. Epub 2015 Feb 23. Semin Cell Dev Biol. 2015. PMID: 25721809 Review.
-
Low-dose radiation-induced epithelial-mesenchymal transition through NF-κB in cervical cancer cells.Int J Oncol. 2013 May;42(5):1801-6. doi: 10.3892/ijo.2013.1852. Epub 2013 Mar 8. Int J Oncol. 2013. PMID: 23483258
Cited by
-
The Effect of Ionising Radiation on the Properties of Tumour-Derived Exosomes and Their Ability to Modify the Biology of Non-Irradiated Breast Cancer Cells-An In Vitro Study.Int J Mol Sci. 2025 Jan 4;26(1):376. doi: 10.3390/ijms26010376. Int J Mol Sci. 2025. PMID: 39796230 Free PMC article.
-
Effect of gold nanoparticles in radiation therapy of breast tumor: photon, electron, proton, neutron, helium, and carbon ions irradiation.Radiol Phys Technol. 2025 Sep;18(3):670-687. doi: 10.1007/s12194-025-00919-w. Epub 2025 Jun 6. Radiol Phys Technol. 2025. PMID: 40478479
-
Ionizing Radiation-Induced Extracellular Vesicle Release Promotes AKT-Associated Survival Response in SH-SY5Y Neuroblastoma Cells.Cells. 2021 Jan 8;10(1):107. doi: 10.3390/cells10010107. Cells. 2021. PMID: 33430027 Free PMC article.
-
Sublethal irradiation promotes the metastatic potential of hepatocellular carcinoma cells.Cancer Sci. 2021 Jan;112(1):265-274. doi: 10.1111/cas.14724. Epub 2020 Nov 29. Cancer Sci. 2021. PMID: 33155388 Free PMC article.
-
New Discoveries in Radiation Science.Cancers (Basel). 2021 Mar 2;13(5):1034. doi: 10.3390/cancers13051034. Cancers (Basel). 2021. PMID: 33801176 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

