Drosophila as a Model Organism to Understand the Effects during Development of TFIIH-Related Human Diseases
- PMID: 31963603
- PMCID: PMC7013941
- DOI: 10.3390/ijms21020630
Drosophila as a Model Organism to Understand the Effects during Development of TFIIH-Related Human Diseases
Abstract
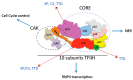
Human mutations in the transcription and nucleotide excision repair (NER) factor TFIIH are linked with three human syndromes: xeroderma pigmentosum (XP), trichothiodystrophy (TTD) and Cockayne syndrome (CS). In particular, different mutations in the XPB, XPD and p8 subunits of TFIIH may cause one or a combination of these syndromes, and some of these mutations are also related to cancer. The participation of TFIIH in NER and transcription makes it difficult to interpret the different manifestations observed in patients, particularly since some of these phenotypes may be related to problems during development. TFIIH is present in all eukaryotic cells, and its functions in transcription and DNA repair are conserved. Therefore, Drosophila has been a useful model organism for the interpretation of different phenotypes during development as well as the understanding of the dynamics of this complex. Interestingly, phenotypes similar to those observed in humans caused by mutations in the TFIIH subunits are present in mutant flies, allowing the study of TFIIH in different developmental processes. Furthermore, studies performed in Drosophila of mutations in different subunits of TFIIH that have not been linked to any human diseases, probably because they are more deleterious, have revealed its roles in differentiation and cell death. In this review, different achievements made through studies in the fly to understand the functions of TFIIH during development and its relationship with human diseases are analysed and discussed.
Keywords: Cancer; Drosophila; TFIIH; development; human syndromes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
p8/TTDA overexpression enhances UV-irradiation resistance and suppresses TFIIH mutations in a Drosophila trichothiodystrophy model.PLoS Genet. 2008 Nov;4(11):e1000253. doi: 10.1371/journal.pgen.1000253. Epub 2008 Nov 14. PLoS Genet. 2008. PMID: 19008953 Free PMC article.
-
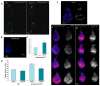
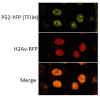
DNA repair and transcriptional deficiencies caused by mutations in the Drosophila p52 subunit of TFIIH generate developmental defects and chromosome fragility.Mol Cell Biol. 2007 May;27(10):3640-50. doi: 10.1128/MCB.00030-07. Epub 2007 Mar 5. Mol Cell Biol. 2007. PMID: 17339330 Free PMC article.
-
Comparative study of nucleotide excision repair defects between XPD-mutated fibroblasts derived from trichothiodystrophy and xeroderma pigmentosum patients.DNA Repair (Amst). 2008 Dec 1;7(12):1990-8. doi: 10.1016/j.dnarep.2008.08.009. Epub 2008 Oct 10. DNA Repair (Amst). 2008. PMID: 18817897
-
TFIIH central activity in nucleotide excision repair to prevent disease.DNA Repair (Amst). 2023 Dec;132:103568. doi: 10.1016/j.dnarep.2023.103568. Epub 2023 Sep 7. DNA Repair (Amst). 2023. PMID: 37977600 Review.
-
Hot topics in DNA repair: the molecular basis for different disease states caused by mutations in TFIIH and XPG.DNA Repair (Amst). 2008 Feb 1;7(2):339-44. doi: 10.1016/j.dnarep.2007.10.007. DNA Repair (Amst). 2008. PMID: 18077223 Free PMC article. Review.
Cited by
-
Moonlighting in Mitosis: Analysis of the Mitotic Functions of Transcription and Splicing Factors.Cells. 2020 Jun 26;9(6):1554. doi: 10.3390/cells9061554. Cells. 2020. PMID: 32604778 Free PMC article. Review.
References
-
- Olson C.M., Liang Y., Leggett A., Park W.D., Li L., Mills C.E., Elsarrag S.Z., Ficarro S.B., Zhang T., Düster R., et al. Development of a Selective CDK7 Covalent Inhibitor Reveals Predominant Cell-Cycle Phenotype. Cell Chem. Biol. 2019;26:792–803. doi: 10.1016/j.chembiol.2019.02.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

