Biodegradability of Dental Care Antimicrobial Agents Chlorhexidine and Octenidine by Ligninolytic Fungi
- PMID: 31963668
- PMCID: PMC7024351
- DOI: 10.3390/molecules25020400
Biodegradability of Dental Care Antimicrobial Agents Chlorhexidine and Octenidine by Ligninolytic Fungi
Abstract
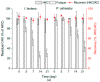
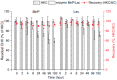
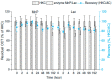
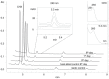
Chlorhexidine (CHX) and octenidine (OCT), antimicrobial compounds used in oral care products (toothpastes and mouthwashes), were recently revealed to interfere with human sex hormone receptor pathways. Experiments employing model organisms-white-rot fungi Irpex lacteus and Pleurotus ostreatus-were carried out in order to investigate the biodegradability of these endocrine-disrupting compounds and the capability of the fungi and their extracellular enzyme apparatuses to biodegrade CHX and OCT. Up to 70% ± 6% of CHX was eliminated in comparison with a heat-killed control after 21 days of in vivo incubation. An additional in vitro experiment confirmed manganese-dependent peroxidase and laccase are partially responsible for the removal of CHX. Up to 48% ± 7% of OCT was removed in the same in vivo experiment, but the strong sorption of OCT on fungal biomass prevented a clear evaluation of the involvement of the fungi or extracellular enzymes. On the other hand, metabolites indicating the enzymatic transformation of both CHX and OCT were detected and their chemical structures were proposed by means of liquid chromatography-mass spectrometry. Complete biodegradation by the ligninolytic fungi was not achieved for any of the studied analytes, which emphasizes their recalcitrant character with low possibility to be removed from the environment.
Keywords: chlorhexidine; dental hygiene; laccase; ligninolytic fungi; manganese-dependent peroxidase; octenidine; personal care products; quaternary ammonium compounds; recalcitrant pollutant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Usage of Mouthwash/dental Rinse in the U.S. 2011–2023. [(accessed on 29 November 2019)]; Available online: https://www.statista.com/statistics/286902/usage-mouthwash-dental-rinse-...
-
- Cesen M., Heath D., Krivec M., Kosmrlj J., Kosjek T., Heath E. Seasonal and spatial variations in the occurrence, mass loadings and removal of compounds of emerging concern in the Slovene aqueous environment and environmental risk assessment. Environ. Pollut. 2018;242:143–154. doi: 10.1016/j.envpol.2018.06.052. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

