Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures
- PMID: 31963682
- PMCID: PMC7024228
- DOI: 10.3390/molecules25020403
Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures
Abstract
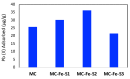
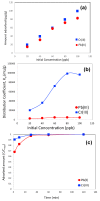
Lead pollution in drinking water is one of the most common problems worldwide. In this research, sulfur and iron dual-doped mesoporous carbons are synthesized by soft-templating with sulfur content 4.4-6.1 atom% and iron content 7.8-9 atom%. Sulfur functionalities of the carbons are expected to enhance the affinity of the carbon toward lead whereas iron content is expected to separate the carbon from water owing to its magnetic properties. All the carbons were characterized by pore textural properties, x-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and energy dispersive x-ray (EDX). In order to study the Pb(II) removal efficiently of this carbon in competitive mode and to mimic the real-world use, one additional heavy-metal, including Cr(III), and four other commonly occurring metals-Na(I), K(I), Ca(II) and Fe (III)-are added with lead prior to adsorption experiments. It was observed that Pb(II) adsorption capacity of this carbon was not influenced by the presence of other metals. A highly elevated concentration of Na(I), K(I), Ca(II) and Fe(III) in the eluting solution compared to the initial dose suggested possible leaching of those metals from other salts as impurities, water source or even from the carbon itself, although the XPS analysis of the carbon confirmed negligible adsorption of those metals in carbon. From the equilibrium and kinetic data of adsorption, few parameters have been calculated, including distribution coefficient, diffusive time constant and pseudosecond order rate constant. The overall results suggest that these iron and sulfur dual-doped mesoporous carbons can serve as potential adsorbents for removal of lead from drinking water in the presence of other competing metals.
Keywords: adsorption; doping; lead; mesoporous carbons; water pollution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Momčilović M., Purenović M., Bojić A., Zarubica A., Ranđelović M. Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination. 2011;276:53–59. doi: 10.1016/j.desal.2011.03.013. - DOI
-
- Gercel O., Gercel H.F. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Eng. J. 2007;132:289–297. doi: 10.1016/j.cej.2007.01.010. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

