Ringer's Lactate Prevents Early Organ Failure by Providing Extracellular Calcium
- PMID: 31963691
- PMCID: PMC7019478
- DOI: 10.3390/jcm9010263
Ringer's Lactate Prevents Early Organ Failure by Providing Extracellular Calcium
Abstract
Objective: Ringer's lactate may improve early systemic inflammation during critical illnesses like severe acute pancreatitis, which are associated with hypocalcemia. Ringer's lactate is buffered and contains lactate and calcium. We, thus analyzed extracellular calcium or lactate's effects on the mechanisms, intermediary markers, and organ failure in models mimicking human disease with nonesterified fatty acid (NEFA) elevation.
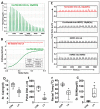
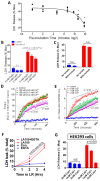
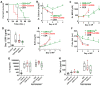
Methods: Meta-analyses and experimental studies were performed. Experimentally, extracellular calcium and lactate were compared in their interaction with linoleic acid (LA; a NEFA increased in human severe pancreatitis), and its subsequent effects on mitochondrial depolarization and cytosolic calcium signaling resulting in cell injury. In vivo, the effect of LA was studied on organ failure, along with the effect of calcium or lactate (pH 7.4) on severe acute pancreatitis-associated organ failure. A meta-analysis of human randomized control trials comparing Ringer's lactate to normal saline was done, focusing on necrosis and organ failure.
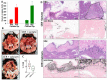
Results: Calcium reacted ionically with LA and reduced lipotoxic necrosis. In vivo, LA induced organ failure and hypocalcemia. During severe pancreatitis, calcium supplementation in saline pH 7.4, unlike lactate, prevented hypocalcemia, increased NEFA saponification, reduced circulating NEFA and C-reactive protein , reduced pancreatic necrosis adjacent to fat necrosis, and normalized shock (carotid pulse distension) and blood urea nitrogen elevation on day 1. This, however, did not prevent the later increase in serum NEFA which caused delayed organ failure. Meta-analysis showed Ringer's lactate reduced necrosis, but not organ failure, compared with normal saline.
Conclusion: Hypocalcemia occurs due to excess NEFA binding calcium during a critical illness. Ringer's lactate's early benefits in systemic inflammation are by the calcium it provides reacting ionically with NEFA. This, however, does not prevent later organ failure from sustained NEFA generation. Future studies comparing calcium supplemented saline resuscitation to Ringer's lactate may provide insights to this pathophysiology.
Keywords: CRP; Ringer’s lactate; calcium; inflammation; isothermal titration calorimetry; lipolysis; mitochondrial depolarization; organ failure; pancreatitis; saponification.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
-
- Mounzer R., Langmead C.J., Wu B.U., Evans A.C., Bishehsari F., Muddana V., Singh V.K., Slivka A., Whitcomb D.C., Yadav D., et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482. doi: 10.1053/j.gastro.2012.03.005. - DOI - PubMed
-
- Ranson J.H., Rifkind K.M., Roses D.F., Fink S.D., Eng K., Spencer F.C. Prognostic signs and the role of operative management in acute pancreatitis. Surg. Gynecol. Obstet. 1974;139:69–81. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

