Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis
- PMID: 31963721
- PMCID: PMC7014203
- DOI: 10.3390/ijms21020644
Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis
Abstract
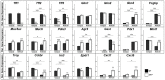
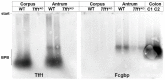
TFF1 is a peptide of the gastric mucosa co-secreted with the mucin MUC5AC. It plays a key role in gastric mucosal protection and repair. Tff1-deficient (Tff1KO) mice obligatorily develop antropyloric adenoma and about 30% progress to carcinomas. Thus, these mice represent a model for gastric tumorigenesis. Here, we compared the expression of selected genes in Tff1KO mice and the corresponding wild-type animals (RT-PCR analyses). Furthermore, we systematically investigated the different molecular forms of Tff1 and its heterodimer partner gastrokine-2 (Gkn2) in the stomach (Western blot analyses). As a hallmark, a large portion of murine Tff1 occurs in a monomeric form. This is unexpected because of its odd number of seven cysteine residues. Probably the three conserved acid amino acid residues (EEE) flanking the 7th cysteine residue allow monomeric secretion. As a consequence, the free thiol of monomeric Tff1 could have a protective scavenger function, e.g., for reactive oxygen/nitrogen species. Furthermore, a minor subset of Tff1 forms a disulfide-linked heterodimer with IgG Fc binding protein (Fcgbp). Of special note, in Tff1KO animals a homodimeric form of Gkn2 was observed. In addition, Tff1KO animals showed strongly reduced Tff2 transcript and protein levels, which might explain their increased sensitivity to Helicobacter pylori infection.
Keywords: FCGBP; ROS; TFF1; TFF2; carcinogenesis; gastric cancer; inflammation; innate immunity; oxidative stress; trefoil factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm.Int J Mol Sci. 2020 Jun 25;21(12):4535. doi: 10.3390/ijms21124535. Int J Mol Sci. 2020. PMID: 32630599 Free PMC article. Review.
-
Expression Profiling along the Murine Intestine: Different Mucosal Protection Systems and Alterations in Tff1-Deficient Animals.Int J Mol Sci. 2023 Aug 11;24(16):12684. doi: 10.3390/ijms241612684. Int J Mol Sci. 2023. PMID: 37628863 Free PMC article.
-
The Tumor Suppressor TFF1 Occurs in Different Forms and Interacts with Multiple Partners in the Human Gastric Mucus Barrier: Indications for Diverse Protective Functions.Int J Mol Sci. 2020 Apr 4;21(7):2508. doi: 10.3390/ijms21072508. Int J Mol Sci. 2020. PMID: 32260357 Free PMC article.
-
Biosynthesis of gastrokine-2 in the human gastric mucosa: restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins.Cell Physiol Biochem. 2007;20(6):899-908. doi: 10.1159/000110450. Cell Physiol Biochem. 2007. PMID: 17982272
-
Trefoil Factor Family (TFF) Peptides and their Different Roles in the Mucosal Innate Immune Defense and More: An Update.Curr Med Chem. 2021;28(36):7387-7399. doi: 10.2174/0929867328666210215114140. Curr Med Chem. 2021. PMID: 33588719 Review.
Cited by
-
Profiling of the Bacterial Microbiota along the Murine Alimentary Tract.Int J Mol Sci. 2022 Feb 4;23(3):1783. doi: 10.3390/ijms23031783. Int J Mol Sci. 2022. PMID: 35163705 Free PMC article.
-
A Novel Prognostic Marker and Therapeutic Target Associated with Glioma Progression in a Tumor Immune Microenvironment.J Inflamm Res. 2023 Mar 1;16:895-916. doi: 10.2147/JIR.S398775. eCollection 2023. J Inflamm Res. 2023. PMID: 36883185 Free PMC article.
-
Elevated Levels of MUC and JADE1 Predict Poor Prognosis of Patients with Gastric Cancer.Cancer Manag Res. 2025 Mar 13;17:577-587. doi: 10.2147/CMAR.S493015. eCollection 2025. Cancer Manag Res. 2025. PMID: 40098804 Free PMC article.
-
Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm.Int J Mol Sci. 2020 Jun 25;21(12):4535. doi: 10.3390/ijms21124535. Int J Mol Sci. 2020. PMID: 32630599 Free PMC article. Review.
-
Expression Profiling along the Murine Intestine: Different Mucosal Protection Systems and Alterations in Tff1-Deficient Animals.Int J Mol Sci. 2023 Aug 11;24(16):12684. doi: 10.3390/ijms241612684. Int J Mol Sci. 2023. PMID: 37628863 Free PMC article.
References
-
- Hoffmann W. TFF peptides. In: Kastin A., editor. Handbook of Biologically Active Peptides. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2013. pp. 1338–1345.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

