MicroRNAs in Vascular Eye Diseases
- PMID: 31963809
- PMCID: PMC7014392
- DOI: 10.3390/ijms21020649
MicroRNAs in Vascular Eye Diseases
Abstract
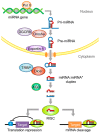
Since the discovery of the first microRNA (miRNA) decades ago, studies of miRNA biology have expanded in many biomedical research fields, including eye research. The critical roles of miRNAs in normal development and diseases have made miRNAs useful biomarkers or molecular targets for potential therapeutics. In the eye, ocular neovascularization (NV) is a leading cause of blindness in multiple vascular eye diseases. Current anti-angiogenic therapies, such as anti-vascular endothelial growth factor (VEGF) treatment, have their limitations, indicating the need for investigating new targets. Recent studies established the roles of various miRNAs in the regulation of pathological ocular NV, suggesting miRNAs as both biomarkers and therapeutic targets in vascular eye diseases. This review summarizes the biogenesis of miRNAs, and their functions in the normal development and diseases of the eye, with a focus on clinical and experimental retinopathies in both human and animal models. Discovery of novel targets involving miRNAs in vascular eye diseases will provide insights for developing new treatments to counter ocular NV.
Keywords: AMD: biomarker; eye disease; microRNA; neovascularization; retinopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
microRNA-181a inhibits ocular neovascularization by interfering with vascular endothelial growth factor expression.Cardiovasc Ther. 2018 Jun;36(3):e12329. doi: 10.1111/1755-5922.12329. Epub 2018 Apr 24. Cardiovasc Ther. 2018. PMID: 29608244
-
Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization.Proc Natl Acad Sci U S A. 2015 Sep 29;112(39):12163-8. doi: 10.1073/pnas.1508426112. Epub 2015 Sep 15. Proc Natl Acad Sci U S A. 2015. PMID: 26374840 Free PMC article.
-
Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy.Prog Retin Eye Res. 2007 Jan;26(1):1-37. doi: 10.1016/j.preteyeres.2006.09.002. Epub 2006 Oct 30. Prog Retin Eye Res. 2007. PMID: 17074526 Review.
-
MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model.PLoS One. 2019 Jun 12;14(6):e0218282. doi: 10.1371/journal.pone.0218282. eCollection 2019. PLoS One. 2019. PMID: 31188886 Free PMC article.
-
MicroRNAs and the HIF/VEGF axis in ocular neovascular diseases.Acta Ophthalmol. 2021 Dec;99(8):e1255-e1262. doi: 10.1111/aos.14845. Epub 2021 Mar 17. Acta Ophthalmol. 2021. PMID: 33729690 Review.
Cited by
-
Aqueous microRNA profiling in age-related macular degeneration and polypoidal choroidal vasculopathy by next-generation sequencing.Sci Rep. 2023 Jan 23;13(1):1274. doi: 10.1038/s41598-023-28385-7. Sci Rep. 2023. PMID: 36690666 Free PMC article.
-
Machine learning uncovers novel sex-specific dementia biomarkers linked to autism and eye diseases.J Alzheimers Dis Rep. 2025 Feb 13;9:25424823251317177. doi: 10.1177/25424823251317177. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40034518 Free PMC article.
-
Differential Circulating MicroRNA Expression in Age-Related Macular Degeneration.Int J Mol Sci. 2021 Nov 15;22(22):12321. doi: 10.3390/ijms222212321. Int J Mol Sci. 2021. PMID: 34830203 Free PMC article.
-
Potential epigenetic molecular regulatory networks in ocular neovascularization.Front Genet. 2022 Sep 2;13:970224. doi: 10.3389/fgene.2022.970224. eCollection 2022. Front Genet. 2022. PMID: 36118885 Free PMC article. Review.
-
MicroRNA-376b-3p Suppresses Choroidal Neovascularization by Regulating Glutaminolysis in Endothelial Cells.Invest Ophthalmol Vis Sci. 2023 Jan 3;64(1):22. doi: 10.1167/iovs.64.1.22. Invest Ophthalmol Vis Sci. 2023. PMID: 36719700 Free PMC article.
References
-
- Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

