Curcumin Affects HSP60 Folding Activity and Levels in Neuroblastoma Cells
- PMID: 31963896
- PMCID: PMC7013437
- DOI: 10.3390/ijms21020661
Curcumin Affects HSP60 Folding Activity and Levels in Neuroblastoma Cells
Abstract
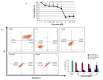
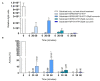
The fundamental challenge in fighting cancer is the development of protective agents able to interfere with the classical pathways of malignant transformation, such as extracellular matrix remodeling, epithelial-mesenchymal transition and, alteration of protein homeostasis. In the tumors of the brain, proteotoxic stress represents one of the main triggering agents for cell transformation. Curcumin is a natural compound with anti-inflammatory and anti-cancer properties with promising potential for the development of therapeutic drugs for the treatment of cancer as well as neurodegenerative diseases. Among the mediators of cancer development, HSP60 is a key factor for the maintenance of protein homeostasis and cell survival. High HSP60 levels were correlated, in particular, with cancer development and progression, and for this reason, we investigated the ability of curcumin to affect HSP60 expression, localization, and post-translational modifications using a neuroblastoma cell line. We have also looked at the ability of curcumin to interfere with the HSP60/HSP10 folding machinery. The cells were treated with 6, 12.5, and 25 µM of curcumin for 24 h, and the flow cytometry analysis showed that the compound induced apoptosis in a dose-dependent manner with a higher percentage of apoptotic cells at 25 µM. This dose of curcumin-induced a decrease in HSP60 protein levels and an upregulation of HSP60 mRNA expression. Moreover, 25 µM of curcumin reduced HSP60 ubiquitination and nitration, and the chaperonin levels were higher in the culture media compared with the untreated cells. Furthermore, curcumin at the same dose was able to favor HSP60 folding activity. The reduction of HSP60 levels, together with the increase in its folding activity and the secretion in the media led to the supposition that curcumin might interfere with cancer progression with a protective mechanism involving the chaperonin.
Keywords: HSP60; brain tumors; extracellular HSP60; heat shock proteins; molecular chaperones; post-translational modifications; protein folding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells.Oncotarget. 2016 May 17;7(20):28849-67. doi: 10.18632/oncotarget.6680. Oncotarget. 2016. PMID: 26700624 Free PMC article.
-
Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence.Cancer Lett. 2017 Jan 28;385:75-86. doi: 10.1016/j.canlet.2016.10.045. Epub 2016 Nov 9. Cancer Lett. 2017. PMID: 27836734
-
CCAR2/DBC1 and Hsp60 Positively Regulate Expression of Survivin in Neuroblastoma Cells.Int J Mol Sci. 2019 Jan 1;20(1):131. doi: 10.3390/ijms20010131. Int J Mol Sci. 2019. PMID: 30609639 Free PMC article.
-
Modulatory effects of curcumin on heat shock proteins in cancer: A promising therapeutic approach.Biofactors. 2019 Sep;45(5):631-640. doi: 10.1002/biof.1522. Epub 2019 May 28. Biofactors. 2019. PMID: 31136038 Review.
-
Molecular chaperone disorders: defective Hsp60 in neurodegeneration.Curr Top Med Chem. 2012;12(22):2491-503. doi: 10.2174/1568026611212220005. Curr Top Med Chem. 2012. PMID: 23339303 Review.
Cited by
-
MiRNAs in Extracellular Vesicles as Biomarkers in Plasma of Papillary Thyroid Cancer Patients: A Proof-of-Concept Study.Biology (Basel). 2024 Sep 22;13(9):743. doi: 10.3390/biology13090743. Biology (Basel). 2024. PMID: 39336170 Free PMC article.
-
Modulating disease-relevant tau oligomeric strains by small molecules.J Biol Chem. 2020 Oct 30;295(44):14807-14825. doi: 10.1074/jbc.RA120.014630. Epub 2020 Jul 31. J Biol Chem. 2020. PMID: 32737202 Free PMC article.
-
Curcumin's Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions.Biomolecules. 2020 Oct 22;10(11):1469. doi: 10.3390/biom10111469. Biomolecules. 2020. PMID: 33105719 Free PMC article. Review.
-
Glutamine sustains energy metabolism and alleviates liver injury in burn sepsis by promoting the assembly of mitochondrial HSP60-HSP10 complex via SIRT4 dependent protein deacetylation.Redox Rep. 2024 Dec;29(1):2312320. doi: 10.1080/13510002.2024.2312320. Epub 2024 Feb 8. Redox Rep. 2024. PMID: 38329114 Free PMC article.
-
Exosomes in glioma: mechanistic insights on biological, therapeutic, and diagnostic perspective.Ther Deliv. 2025 May;16(5):475-486. doi: 10.1080/20415990.2025.2466410. Epub 2025 Feb 16. Ther Deliv. 2025. PMID: 39957239 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

