Opportunities for an atherosclerosis vaccine: From mice to humans
- PMID: 31964554
- PMCID: PMC7939143
- DOI: 10.1016/j.vaccine.2019.12.039
Opportunities for an atherosclerosis vaccine: From mice to humans
Abstract
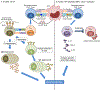
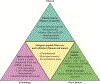
Atherosclerosis, the major underlying cause of cardiovascular diseases (CVD), is the number one killer globally. The disease pathogenesis involves a complex interplay between metabolic and immune components. Although lipid-lowering drugs such as statins curb the risks associated with CVD, significant residual inflammatory risk remains. Substantial evidence from experimental models and clinical studies has established the role of inflammation and immune effector mechanisms in the pathogenesis of atherosclerosis. Several stages of the disease are affected by host-mediated antigen-specific adaptive immune responses that play either protective or proatherogenic roles. Therefore, strategies to boost an anti-atherogenic humoral and T regulatory cell response are emerging as preventative or therapeutic strategies to lowering inflammatory residual risks. Vaccination holds promise as an efficient, durable and relatively inexpensive approach to induce protective adaptive immunity in atherosclerotic patients. In this review, we discuss the status and opportunities for a human atherosclerosis vaccine. We describe (1) some of the immunomodulatory therapeutic interventions tested in atherosclerosis (2) the immune targets identified in pre-clinical and clinical investigations (3) immunization strategies evaluated in animal models (4) past and ongoing clinical trials to examine the safety and efficacy of human atherosclerosis vaccines and (5) strategies to improve and optimize vaccination in humans (antigen selection, formulation, dose and delivery).
Keywords: Antigen-specific; Atherosclerosis; Immunomodulation; Peptide-based vaccine; Tregs.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. - PubMed
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–31. - PubMed
-
- Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, et al. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

