Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study
- PMID: 31964641
- PMCID: PMC7190062
- DOI: 10.1136/bmj.l6802
Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study
Erratum in
-
Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study.BMJ. 2020 Feb 5;368:m358. doi: 10.1136/bmj.m358. BMJ. 2020. PMID: 32024659 No abstract available.
Abstract
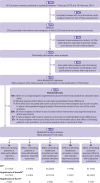
Objectives: To study the impact of blinding on estimated treatment effects, and their variation between trials; differentiating between blinding of patients, healthcare providers, and observers; detection bias and performance bias; and types of outcome (the MetaBLIND study).
Design: Meta-epidemiological study.
Data source: Cochrane Database of Systematic Reviews (2013-14).
Eligibility criteria for selecting studies: Meta-analyses with both blinded and non-blinded trials on any topic.
Review methods: Blinding status was retrieved from trial publications and authors, and results retrieved automatically from the Cochrane Database of Systematic Reviews. Bayesian hierarchical models estimated the average ratio of odds ratios (ROR), and estimated the increases in heterogeneity between trials, for non-blinded trials (or of unclear status) versus blinded trials. Secondary analyses adjusted for adequacy of concealment of allocation, attrition, and trial size, and explored the association between outcome subjectivity (high, moderate, low) and average bias. An ROR lower than 1 indicated exaggerated effect estimates in trials without blinding.
Results: The study included 142 meta-analyses (1153 trials). The ROR for lack of blinding of patients was 0.91 (95% credible interval 0.61 to 1.34) in 18 meta-analyses with patient reported outcomes, and 0.98 (0.69 to 1.39) in 14 meta-analyses with outcomes reported by blinded observers. The ROR for lack of blinding of healthcare providers was 1.01 (0.84 to 1.19) in 29 meta-analyses with healthcare provider decision outcomes (eg, readmissions), and 0.97 (0.64 to 1.45) in 13 meta-analyses with outcomes reported by blinded patients or observers. The ROR for lack of blinding of observers was 1.01 (0.86 to 1.18) in 46 meta-analyses with subjective observer reported outcomes, with no clear impact of degree of subjectivity. Information was insufficient to determine whether lack of blinding was associated with increased heterogeneity between trials. The ROR for trials not reported as double blind versus those that were double blind was 1.02 (0.90 to 1.13) in 74 meta-analyses.
Conclusion: No evidence was found for an average difference in estimated treatment effect between trials with and without blinded patients, healthcare providers, or outcome assessors. These results could reflect that blinding is less important than often believed or meta-epidemiological study limitations, such as residual confounding or imprecision. At this stage, replication of this study is suggested and blinding should remain a methodological safeguard in trials.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Blindsided: challenging the dogma of masking in clinical trials.BMJ. 2020 Jan 21;368:m229. doi: 10.1136/bmj.m229. BMJ. 2020. PMID: 32020896 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
