Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts
- PMID: 31964734
- PMCID: PMC6974568
- DOI: 10.1128/mBio.02956-19
Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts
Erratum in
-
Erratum for Dubrovsky et al., "Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts".mBio. 2020 Mar 17;11(2):e00234-20. doi: 10.1128/mBio.00234-20. mBio. 2020. PMID: 32184240 Free PMC article. No abstract available.
Abstract
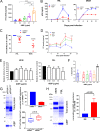
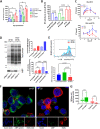
Apolipoprotein A-I binding protein (AIBP) is a protein involved in regulation of lipid rafts and cholesterol efflux. AIBP has been suggested to function as a protective factor under several sets of pathological conditions associated with increased abundance of lipid rafts, such as atherosclerosis and acute lung injury. Here, we show that exogenously added AIBP reduced the abundance of lipid rafts and inhibited HIV replication in vitro as well as in HIV-infected humanized mice, whereas knockdown of endogenous AIBP increased HIV replication. Endogenous AIBP was much more abundant in activated T cells than in monocyte-derived macrophages (MDMs), and exogenous AIBP was much less effective in T cells than in MDMs. AIBP inhibited virus-cell fusion, specifically targeting cells with lipid rafts mobilized by cell activation or Nef-containing exosomes. MDM-HIV fusion was sensitive to AIBP only in the presence of Nef provided by the virus or exosomes. Peripheral blood mononuclear cells from donors with the HLA-B*35 genotype, associated with rapid progression of HIV disease, bound less AIBP than cells from donors with other HLA genotypes and were not protected by AIBP from rapid HIV-1 replication. These results provide the first evidence for the role of Nef exosomes in regulating HIV-cell fusion by modifying lipid rafts and suggest that AIBP is an innate factor that restricts HIV replication by targeting lipid rafts.IMPORTANCE Apolipoprotein A-I binding protein (AIBP) is a recently identified innate anti-inflammatory factor. Here, we show that AIBP inhibited HIV replication by targeting lipid rafts and reducing virus-cell fusion. Importantly, AIBP selectively reduced levels of rafts on cells stimulated by an inflammatory stimulus or treated with extracellular vesicles containing HIV-1 protein Nef without affecting rafts on nonactivated cells. Accordingly, fusion of monocyte-derived macrophages with HIV was sensitive to AIBP only in the presence of Nef. Silencing of endogenous AIBP significantly upregulated HIV-1 replication. Interestingly, HIV-1 replication in cells from donors with the HLA-B*35 genotype, associated with rapid progression of HIV disease, was not inhibited by AIBP. These results suggest that AIBP is an innate anti-HIV factor that targets virus-cell fusion.
Keywords: AIBP; HIV; HLA; Nef; exosomes; extracellular vesicles; fusion; lipid rafts.
Copyright © 2020 Dubrovsky et al.
Figures



References
-
- Ritter M, Buechler C, Boettcher A, Barlage S, Schmitz-Madry A, Orso E, Bared SM, Schmiedeknecht G, Baehr CH, Fricker G, Schmitz G. 2002. Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I. Genomics 79:693–702. doi:10.1006/geno.2002.6761. - DOI - PubMed
-
- Gu Q, Yang X, Lv J, Zhang J, Xia B, Kim JD, Wang R, Xiong F, Meng S, Clements TP, Tandon B, Wagner DS, Diaz MF, Wenzel PL, Miller YI, Traver D, Cooke JP, Li W, Zon LI, Chen K, Bai Y, Fang L. 2019. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science 363:1085–1088. doi:10.1126/science.aav1749. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

