Investigating Chaperonin-Containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis
- PMID: 31964905
- PMCID: PMC6972895
- DOI: 10.1038/s41598-020-57602-w
Investigating Chaperonin-Containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis
Abstract
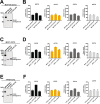
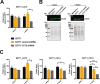
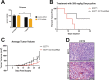
Chaperonin-containing TCP-1 (CCT or TRiC) is a multi-subunit complex that folds many of the proteins essential for cancer development. CCT is expressed in diverse cancers and could be an ideal therapeutic target if not for the fact that the complex is encoded by eight distinct genes, complicating the development of inhibitors. Few definitive studies addressed the role of specific subunits in promoting the chaperonin's function in cancer. To this end, we investigated the activity of CCT2 (CCTβ) by overexpressing or depleting the subunit in breast epithelial and breast cancer cells. We found that increasing total CCT2 in cells by 1.3-1.8-fold using a lentiviral system, also caused CCT3, CCT4, and CCT5 levels to increase. Likewise, silencing cct2 gene expression by ~50% caused other CCT subunits to decrease. Cells expressing CCT2 were more invasive and had a higher proliferative index. CCT2 depletion in a syngeneic murine model of triple negative breast cancer (TNBC) prevented tumor growth. These results indicate that the CCT2 subunit is integral to the activity of the chaperonin and is needed for tumorigenesis. Hence CCT2 could be a viable target for therapeutic development in breast and other cancers.
Conflict of interest statement
A.R.K. is shareholder in Seva Therapeutics, Inc. The other authors have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

