Biology and pathogenesis of human osteosarcoma
- PMID: 31966039
- PMCID: PMC6955653
- DOI: 10.3892/ol.2019.11229
Biology and pathogenesis of human osteosarcoma
Abstract
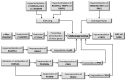
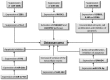
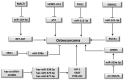
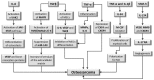
Osteosarcoma (OS) is a bone tumor of mesenchymal origin, most frequently occurring during the rapid growth phase of long bones, and usually located in the epiphyseal growth plates of the femur or the tibia. Its most common feature is genome disorganization, aneuploidy with chromosomal alterations, deregulation of tumor suppressor genes and of the cell cycle, and an absence of DNA repair. This suggests the involvement of surveillance failures, DNA repair or apoptosis control during osteogenesis, allowing the survival of cells which have undergone alterations during differentiation. Epigenetic events, including DNA methylation, histone modifications, nucleosome remodeling and expression of non-coding RNAs have been identified as possible risk factors for the tumor. It has been reported that p53 target genes or those genes that have their activity modulated by p53, in addition to other tumor suppressor genes, are silenced in OS-derived cell lines by hypermethylation of their promoters. In osteogenesis, osteoblasts are formed from pluripotent mesenchymal cells, with potential for self-renewal, proliferation and differentiation into various cell types. This involves complex signaling pathways and multiple factors. Any disturbance in this process can cause deregulation of the differentiation and proliferation of these cells, leading to the malignant phenotype. Therefore, the origin of OS seems to be multifactorial, involving the deregulation of differentiation of mesenchymal cells and tumor suppressor genes, activation of oncogenes, epigenetic events and the production of cytokines.
Keywords: bone tumor; osteosarcoma; osteosarcoma biology; osteosarcoma pathogenesis.
Copyright: © de Azevedo et al.
Figures
References
-
- Stiller CA, Desandes E, Danon SE, Izarzugaza I, Ratiu A, Vassileva-Valerianova Z, Steliarova-Foucher E. Cancer incidence and survival in European adolescents (1978–1997)-report from the automated childhood cancer information system project. Eur J Cancer. 2006;42:2006–2018. doi: 10.1016/j.ejca.2006.05.011. - DOI - PubMed
-
- Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
