Protective effects of neurotensins on lipopolysaccaride-induced acute lung injury by blocking tachykinin mediated pathway
- PMID: 31966569
- PMCID: PMC6965238
Protective effects of neurotensins on lipopolysaccaride-induced acute lung injury by blocking tachykinin mediated pathway
Abstract
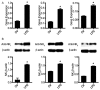
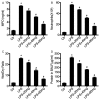
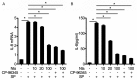
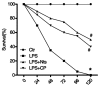
Neurotensin, a bioactive tridecapeptide, has been shown to regulate inflammatory process in lung tissues. However, the effect of neurotensin on LPS-induced lung injury and underlying detailed molecular mechanisms has not been studied. The aim of present study is to investigate the effect of neurotensin on LPS-induced acute lung injury in mice. Mice were treated with LPS intratracheally to induce acute lung injury. 1 hour after ALI induction, and then mice were treated with neurotensins (NTs) (20 mg/kg, 40 mg/kg, and 80 mg/kg) via tail vein injection. Next, the severity of lung injury, MPO activity, neutrophils infiltration, lung edema, protein and pro-inflammatory cytokines concentration in BALF were determined to evaluate the effect of Nts on ALI. Additionally, the expression of tachykinins receptors, including NK1, NK2, and NK3 and the production of IL-8, COX-2, and PGE2 mediated by tachykinins-tachykinins receptors pathway were determined to investigate the blocking effect of Nts on tachykinins and its receptors pathway. Neurotensins treatment significantly decreased the lung edema and the infiltration of inflammatory cells into lung tissue caused by LPS induction. Meanwhile, the elevation of pro-inflammatory cytokines and chemokine in BALF was dramatically reduced by neurotensins treatment. Furthermore, neurotensins could interact with tachykinins receptors and block the inflammatory responses activated by tachykinins pathways. In summary, neurotensins has a potentially protective effect on LPS-induced acute lung injury through the interaction with tachykinins receptors and subsequently blocking the inflammatory responses induced by activation of tachykinins pathway.
Keywords: COX-2; Neurotensin; PGE2; acute lung injury; inflammation; tachykinins.
IJCEP Copyright © 2017.
Conflict of interest statement
None.
Figures



References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. - PubMed
-
- Cannon JG, Tompkins RG, Gelfand JA, Michie HR, Stanford GG, van der Meer JW, Endres S, Lonnemann G, Corsetti J, Chernow B, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
