Physiology and pathology of T-cell aging
- PMID: 31967307
- PMCID: PMC7150735
- DOI: 10.1093/intimm/dxaa006
Physiology and pathology of T-cell aging
Abstract
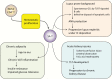
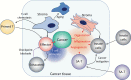
Acquired immune function shows recognizable changes over time with organismal aging. These changes include T-cell dysfunction, which may underlie diminished resistance to infection and possibly various chronic age-associated diseases in the elderly. T-cell dysfunction may occur at distinct stages, from naive cells to the end stages of differentiation during immune responses. The thymus, which generates naive T cells, shows unusually early involution resulting in progressive reduction of T-cell output after adolescence, but peripheral T-cell numbers are maintained through antigen-independent homeostatic proliferation of naive T cells driven by the major histocompatibility complex associated with self-peptides and homeostatic cytokines, retaining the diverse repertoire. However, extensive homeostatic proliferation may lead to the emergence of dysfunctional CD4+ T cells with features resembling senescent cells, termed senescence-associated T (SA-T) cells, which increase and accumulate with age. In situations such as chronic viral infection, T-cell dysfunction may also develop via persistent antigen stimulation, termed exhaustion, preventing possible immunopathology due to excessive immune responses. Exhausted T cells are developed through the effects of checkpoint receptors such as PD-1 and may be reversed with the receptor blockade. Of note, although defective in their regular T-cell antigen-receptor-mediated proliferation, SA-T cells secrete abundant pro-inflammatory factors such as osteopontin, reminiscent of an SA-secretory phenotype. A series of experiments in mouse models indicated that SA-T cells are involved in systemic autoimmunity as well as chronic tissue inflammation following tissue stresses. In this review, we discuss the physiological aspects of T-cell dysfunction associated with aging and its potential pathological involvement in age-associated diseases and possibly cancer.
Keywords: T-cell dysfunction; T-cell exhaustion; age-associated diseases; homeostatic proliferation; senescence-associated T cells.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures

References
-
- Franceschi, C. and Campisi, J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69(Suppl. 1):S4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

