A high-fat diet reverses metabolic disorders and premature aging by modulating insulin and IGF1 signaling in SIRT6 knockout mice
- PMID: 31967391
- PMCID: PMC7059164
- DOI: 10.1111/acel.13104
A high-fat diet reverses metabolic disorders and premature aging by modulating insulin and IGF1 signaling in SIRT6 knockout mice
Abstract
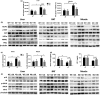
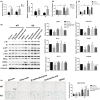
Mammalian sirtuin 6 (SIRT6) is involved in the regulation of many essential processes, especially metabolic homeostasis. SIRT6 knockout mice undergo premature aging and die at age ~4 weeks. Severe glycometabolic disorders have been found in SIRT6 knockout mice, and whether a dietary intervention can rescue SIRT6 knockout mice remains unknown. In our study, we found that at the same calorie intake, a high-fat diet dramatically increased the lifespan of SIRT6 knockout mice to 26 weeks (males) and 37 weeks (females), reversed multi-organ atrophy, and reduced body weight, hypoglycemia, and premature aging. Furthermore, the high-fat diet partially but significantly normalized the global gene expression profile in SIRT6 knockout mice. Regarding the mechanism, excessive glucose uptake and glycolysis induced by the SIRT6 deficiency were attenuated in skeletal muscle through inhibition of insulin and IGF1 signaling by the high-fat diet. Similarly, fatty acids but not ketone bodies inhibited glucose uptake, glycolysis, and senescence in SIRT6 knockout fibroblasts, whereas PI3K inhibition antagonized the effects of a high-fatty-acid medium in vitro. Overall, the high-fat diet dramatically reverses numerous consequences of SIRT6 deficiency through modulation of insulin and IGF1 signaling, providing a new basis for elucidation of SIRT6 and fatty-acid functions and supporting novel therapeutic approaches against metabolic disorders and aging-related diseases.
Keywords: SIRT6; fatty acid; glycolysis; high-fat diet; insulin/IGF1 signaling; organ atrophy.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

