Nanoporous 6H-SiC Photoanodes with a Conformal Coating of Ni-FeOOH Nanorods for Zero-Onset-Potential Water Splitting
- PMID: 31967447
- PMCID: PMC7307839
- DOI: 10.1021/acsami.9b17170
Nanoporous 6H-SiC Photoanodes with a Conformal Coating of Ni-FeOOH Nanorods for Zero-Onset-Potential Water Splitting
Abstract
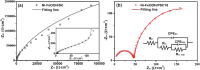
A surface-nanostructured semiconductor photoelectrode is highly desirable for photoelectrochemical (PEC) solar-to-fuel production due to its large active surface area, efficient light absorption, and significantly reduced distance for charge transport. Here, we demonstrate a facile approach to fabricate a nanoporous 6H-silicon carbide (6H-SiC) photoanode with a conformal coating of Ni-FeOOH nanorods as a water oxidation cocatalyst. Such a nanoporous photoanode shows significantly enhanced photocurrent density (jph) with a zero-onset potential. A dendritic porous 6H-SiC with densely arranged holes with a size of ∼40 nm on the surface is fabricated by an anodization method, followed by the hydrothermal deposition of FeOOH nanorods and electrodeposition of NiOOH. Under an illumination of AM1.5G 100 mW/cm2, the Ni-FeOOH-coated nanoporous 6H-SiC photoanode exhibits an onset potential of 0 V versus the reversible hydrogen electrode (VRHE) and a high jph of 0.684 mA/cm2 at 1 VRHE, which is 342 times higher than that of the Ni-FeOOH-coated planar 6H-SiC photoanode. Moreover, the nanoporous photoanode shows a maximum applied-bias-photon-to-current efficiency (ABPE) of 0.58% at a very low bias of 0.36 VRHE, distinctly outperforming the planar counterpart. The impedance measurements demonstrate that the nanoporous photoanode possesses a significantly reduced charge-transfer resistance, which explains the dramatically enhanced PEC water-splitting performance. The reported approach here can be widely used to fabricate other nanoporous semiconductors for solar energy conversion.
Keywords: Ni−FeOOH nanorods; nanoporous silicon carbide; photoelectrochemical water splitting; water oxidation cocatalyst; zero-onset potential.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Zhang Y.; Wang C. W.; Hou H. S.; Zou G. Q.; Ji X. B. Nitrogen Doped/Carbon Tuning Yolk-Like TiO2 and Its Remarkable Impact on Sodium Storage Performances. Adv. Energy Mater. 2017, 7, 160017310.1002/aenm.201600173. - DOI

