N-Heterocyclic Carbene Complexes in C-H Activation Reactions
- PMID: 31967451
- PMCID: PMC7241961
- DOI: 10.1021/acs.chemrev.9b00634
N-Heterocyclic Carbene Complexes in C-H Activation Reactions
Abstract
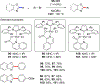
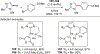
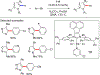
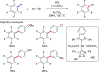
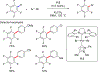
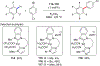
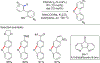
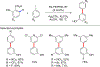
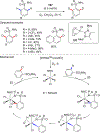
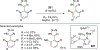
In this contribution, we provide a comprehensive overview of C-H activation methods promoted by NHC-transition metal complexes, covering the literature since 2002 (the year of the first report on metal-NHC-catalyzed C-H activation) through June 2019, focusing on both NHC ligands and C-H activation methods. This review covers C-H activation reactions catalyzed by group 8 to 11 NHC-metal complexes. Through discussing the role of NHC ligands in promoting challenging C-H activation methods, the reader is provided with an overview of this important area and its crucial role in forging carbon-carbon and carbon-heteroatom bonds by directly engaging ubiquitous C-H bonds.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

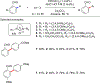
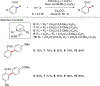
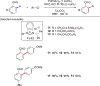
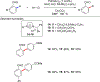



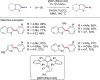



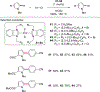

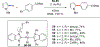
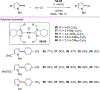
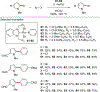
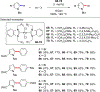
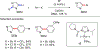
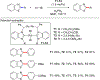
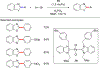
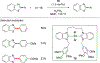
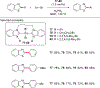


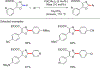
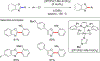


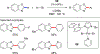
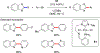
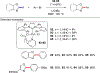
















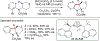
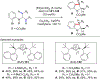
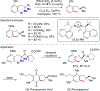
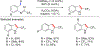






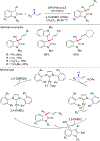


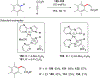

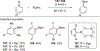
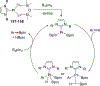


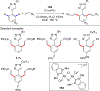
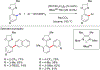
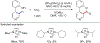


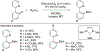

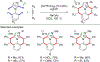





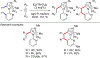
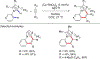
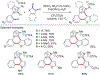
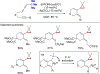
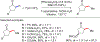
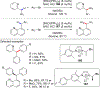

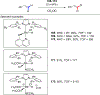
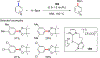
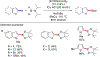
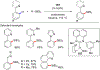
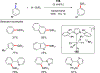



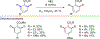
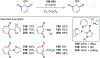

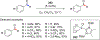
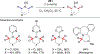


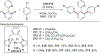

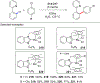

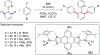
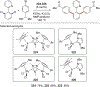

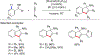


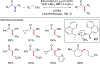
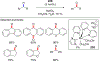

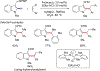




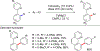
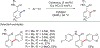










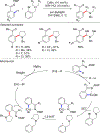



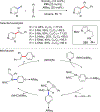



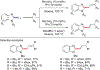

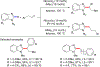
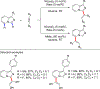
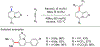
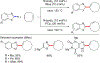


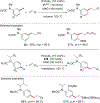


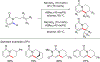
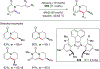
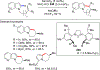
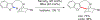

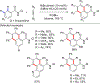
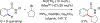


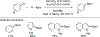

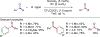
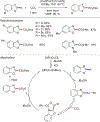
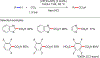


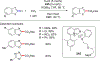


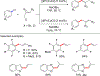


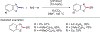




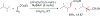



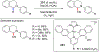
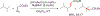
References
-
- Nolan SP N-Heterocyclic Carbenes in Synthesis; 1st Ed, Wiley: New York, 2006.
-
- Nolan SP N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; 1st Ed, Wiley-VCH: Mannheim, 2014.
-
- Nolan S; Cazin C N-Heterocyclic Carbenes in Catalytic Organic Synthesis; 1st Ed, Thieme: Stuttgart, 2017.
-
- Glorius F N-Heterocyclic Carbenes in Transition Metal Catalysis; 1st Ed., Springer: Berlin, 2007.
-
- Huynh HV The Organometallic Chemistry of N-Heterocyclic Carbenes; 1st Ed, John Wiley & Sons: Hoboken, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

