Neural effects of acute stress on appetite: A magnetoencephalography study
- PMID: 31968008
- PMCID: PMC6975544
- DOI: 10.1371/journal.pone.0228039
Neural effects of acute stress on appetite: A magnetoencephalography study
Abstract
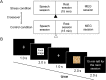
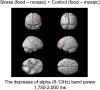
Stress is prevalent in modern society and can affect human health through its effects on appetite. Therefore, in the present study, we aimed to clarify the neural mechanisms by which acute stress affects appetite in healthy, non-obese males during fasting. In total, 22 volunteers participated in two experiments (stress and control conditions) on different days. The participants performed a stress-inducing speech-and-mental-arithmetic task under both conditions, and then viewed images of food, during which, their neural activity was recorded using magnetoencephalography (MEG). In the stress condition, the participants were told to perform the speech-and-mental-arithmetic task again subsequently to viewing the food images; however, another speech-and-mental-arithmetic task was not performed actually. Subjective levels of stress and appetite were then assessed using a visual analog scale. Electrocardiography was performed to assess the index of heart rate variability reflecting sympathetic nerve activity. The findings showed that subjective levels of stress and sympathetic nerve activity were increased in the MEG session in the stress condition, whereas appetite gradually increased in the MEG session only in the control condition. The decrease in alpha band power in the frontal pole caused by viewing the food images was greater in the stress condition than in the control condition. These findings suggest that acute stress can suppress the increase of appetite, and this suppression is associated with the frontal pole. The results of the present study may provide valuable clues to gain a further understanding of the neural mechanisms by which acute stress affects appetite. However, since the stress examined in the present study was related to the expectation of forthcoming stressful event, our present findings may not be generalized to the stress unrelated to the expectation of forthcoming stressful event.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- McCann L, Hughes CM, Adair CG, Cardwell C. Assessing job satisfaction and stress among pharmacists in Northern Ireland. Pharmacy world & science: PWS. 2009;31(2):188–94. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

