Inducible epithelial resistance against acute Sendai virus infection prevents chronic asthma-like lung disease in mice
- PMID: 31968123
- PMCID: PMC7174884
- DOI: 10.1111/bph.14977
Inducible epithelial resistance against acute Sendai virus infection prevents chronic asthma-like lung disease in mice
Abstract
Background and purpose: Respiratory viral infections play central roles in the initiation, exacerbation and progression of asthma in humans. An acute paramyxoviral infection in mice can cause a chronic lung disease that resembles human asthma. We sought to determine whether reduction of Sendai virus lung burden in mice by stimulating innate immunity with aerosolized Toll-like receptor (TLR) agonists could attenuate the severity of chronic asthma-like lung disease.
Experimental approach: Mice were treated by aerosol with 1-μM oligodeoxynucleotide (ODN) M362, an agonist of the TLR9 homodimer, and 4-μM Pam2CSK4 (Pam2), an agonist of the TLR2/6 heterodimer, within a few days before or after Sendai virus challenge.
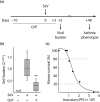
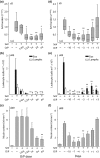
Key results: Treatment with ODN/Pam2 caused ~75% reduction in lung Sendai virus burden 5 days after challenge. The reduction in acute lung virus burden was associated with marked reductions 49 days after viral challenge in eosinophilic and lymphocytic lung inflammation, airway mucous metaplasia, lumenal mucus occlusion and hyperresponsiveness to methacholine. Mechanistically, ODN/Pam2 treatment attenuated the chronic asthma phenotype by suppressing IL-33 production by type 2 pneumocytes, both by reducing the severity of acute infection and by down-regulating Type 2 (allergic) inflammation.
Conclusion and implications: These data suggest that treatment of susceptible human hosts with aerosolized ODN and Pam2 at the time of a respiratory viral infection might attenuate the severity of the acute infection and reduce initiation, exacerbation and progression of asthma.
© 2020 The British Pharmacological Society.
Conflict of interest statement
S.E.E., M.J.T. and B.F.D. are inventors on US patent 8,883,174 “Compositions for Stimulation of Mammalian Innate Immune Resistance to Pathogens,” which has been licensed by their employer, the University of Texas MD Anderson Cancer Center, to Pulmotect, Inc., which is developing O/P as a therapeutic for respiratory infections. In addition, B.L.S., S.E.E., M.J.T. and B.F.D. hold equity in Pulmotect, Inc. and B.L.S. is employed by Pulmotect, Inc.
Figures





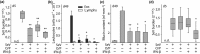
References
-
- Alfaro, V. Y. , Goldblatt, D. L. , Valverde, G. R. , Munsell, M. , Quinton, L. J. , Walker, A. K. , … Dickey, B. F. (2014). Safety, tolerability, and biomarkers of the treatment of mice with aerosolized Toll‐like receptor ligands. Frontiers in Pharmacology, 5(8). 10.3389/fphar.2014.00008 - DOI - PMC - PubMed

