Giant virus diversity and host interactions through global metagenomics
- PMID: 31968354
- PMCID: PMC7162819
- DOI: 10.1038/s41586-020-1957-x
Giant virus diversity and host interactions through global metagenomics
Abstract
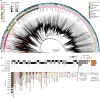
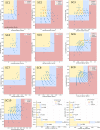
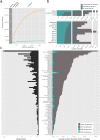
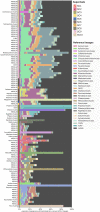
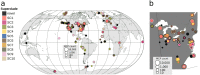
Our current knowledge about nucleocytoplasmic large DNA viruses (NCLDVs) is largely derived from viral isolates that are co-cultivated with protists and algae. Here we reconstructed 2,074 NCLDV genomes from sampling sites across the globe by building on the rapidly increasing amount of publicly available metagenome data. This led to an 11-fold increase in phylogenetic diversity and a parallel 10-fold expansion in functional diversity. Analysis of 58,023 major capsid proteins from large and giant viruses using metagenomic data revealed the global distribution patterns and cosmopolitan nature of these viruses. The discovered viral genomes encoded a wide range of proteins with putative roles in photosynthesis and diverse substrate transport processes, indicating that host reprogramming is probably a common strategy in the NCLDVs. Furthermore, inferences of horizontal gene transfer connected viral lineages to diverse eukaryotic hosts. We anticipate that the global diversity of NCLDVs that we describe here will establish giant viruses-which are associated with most major eukaryotic lineages-as important players in ecosystems across Earth's biomes.
Conflict of interest statement
The authors declare no competing interests.
Figures
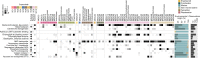






Comment in
-
Learning the diversity of giant viruses.Nat Methods. 2020 Mar;17(3):253. doi: 10.1038/s41592-020-0778-z. Nat Methods. 2020. PMID: 32132722 No abstract available.
References
-
- Abergel C, Legendre M, Claverie J-M. The rapidly expanding universe of giant viruses: mimivirus, pandoravirus, pithovirus and mollivirus. FEMS Microbiol. Rev. 2015;39:779–796. - PubMed
-
- Koonin EV, Yutin N. Evolution of the large nucleocytoplasmic DNA viruses of eukaryotes and convergent origins of viral gigantism. Adv. Virus Res. 2019;103:167–202. - PubMed
-
- Fischer MG. Giant viruses come of age. Curr. Opin. Microbiol. 2016;31:50–57. - PubMed

