Comparison of Allen Carr's Easyway programme with a specialist behavioural and pharmacological smoking cessation support service: a randomized controlled trial
- PMID: 31968400
- PMCID: PMC7186816
- DOI: 10.1111/add.14897
Comparison of Allen Carr's Easyway programme with a specialist behavioural and pharmacological smoking cessation support service: a randomized controlled trial
Abstract
Background and aims: A combination of behavioural and pharmacological support is judged to be the optimal approach for assisting smoking cessation. Allen Carr's Easyway (ACE) is a single-session pharmacotherapy-free programme that has been in operation internationally for 38 years. We compared the effectiveness of ACE with specialist behavioural and pharmacological support delivered to the national standard in England.
Design: A two-arm, parallel-group, single-blind, randomized controlled trial.
Setting: London, UK, between February 2017 and May 2018.
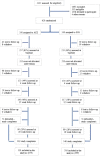
Participants: A total of 620 participants (310 in ACE and 310 in the combined behavioural and pharmacological support condition) were included in the analysis. Adult (≥ 18 years) smokers wanting to quit were randomized in a 1 : 1 ratio. Mean age for the total sample was 40.8 years, with 53.4% being male. Participant baseline characteristics (ethnicity, educational level, number of previous quit attempts, nicotine dependence) were evenly balanced between treatment groups.
Intervention and comparator: The intervention was the ACE method of stopping smoking. This centres on a 4.5-6-hour session of group-based support, alongside subsequent text messages and top-up sessions if needed. It aims to make it easy to stop smoking by convincing smokers that smoking provides no benefits for them. The comparator was a specialist stop smoking service (SSS) providing behavioural and pharmacological support in accordance with national standards.
Measurements: The primary outcome was self-reported continuous abstinence for 26 weeks from the quit/quit re-set date verified by exhaled breath carbon monoxide measurement < 10 parts per million (p.p.m.). Primary analysis was by intention to treat. Secondary outcomes were: use of pharmacotherapy, adverse events and continuous abstinence up to 4 and 12 weeks.
Findings: A total of 468 participants attended treatment (255 ACE versus 213 SSS, P < 0.05). Of those who did attend treatment, 100 completed 6-month measures (23.7% ACE versus 20.7% SSS). Continuous abstinence to 26 weeks was 19.4% (60 of 310) in the ACE intervention and 14.8% (46 of 310) in the SSS intervention [risk difference for ACE versus SSS 4.5% (95% confidence interval (CI) = -1.4 to 10.4%, odds ratio (OR) = 1.38)]. The Bayes factor for superiority of the ACE condition was 1.24.
Conclusion: There was no clear evidence of a difference in the efficacies of the Allen Carr's Easyway (ACE) and specialist smoking cessation support involving behavioural support and pharmacotherapy.
Keywords: Allen Carr; NHS; cessation; randomised controlled trial; smoking.
© 2020 The Authors. Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures
Similar articles
-
The effectiveness of Allen Carr's method for smoking cessation: A systematic review.Tob Prev Cessat. 2023 Sep 29;9:29. doi: 10.18332/tpc/172314. eCollection 2023. Tob Prev Cessat. 2023. PMID: 37780488 Free PMC article. Review.
-
Study protocol for a randomised controlled trial of Allen Carr's Easyway programme versus Lambeth and Southwark NHS for smoking cessation.BMJ Open. 2017 Dec 14;7(12):e016867. doi: 10.1136/bmjopen-2017-016867. BMJ Open. 2017. PMID: 29247083 Free PMC article. Clinical Trial.
-
A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers.Health Technol Assess. 2014 Jan;18(4):1-324. doi: 10.3310/hta18040. Health Technol Assess. 2014. PMID: 24433837 Free PMC article. Clinical Trial.
-
Motivational support intervention to reduce smoking and increase physical activity in smokers not ready to quit: the TARS RCT.Health Technol Assess. 2023 Mar;27(4):1-277. doi: 10.3310/KLTG1447. Health Technol Assess. 2023. PMID: 37022933 Free PMC article.
-
Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial.Lancet Psychiatry. 2019 May;6(5):379-390. doi: 10.1016/S2215-0366(19)30047-1. Epub 2019 Apr 8. Lancet Psychiatry. 2019. PMID: 30975539 Free PMC article. Clinical Trial.
Cited by
-
The effectiveness of Allen Carr's method for smoking cessation: A systematic review.Tob Prev Cessat. 2023 Sep 29;9:29. doi: 10.18332/tpc/172314. eCollection 2023. Tob Prev Cessat. 2023. PMID: 37780488 Free PMC article. Review.
-
The effectiveness of a motivational text-messaging program for smoking cessation after coronary angioplasty: a quasi-experimental study.BMC Res Notes. 2023 Jan 2;16(1):1. doi: 10.1186/s13104-022-06267-x. BMC Res Notes. 2023. PMID: 36593527 Free PMC article. Clinical Trial.
-
Feasibility of a Stop Smoking Program for Healthcare Workers in an Italian Hospital: Econometric Analysis in a Total Worker Health® Approach.Ann Glob Health. 2023 Aug 30;89(1):56. doi: 10.5334/aogh.4153. eCollection 2023. Ann Glob Health. 2023. PMID: 37663224 Free PMC article.
-
Prevalence of Popular Smoking Cessation Aids in England and Associations With Quit Success.JAMA Netw Open. 2025 Jan 2;8(1):e2454962. doi: 10.1001/jamanetworkopen.2024.54962. JAMA Netw Open. 2025. PMID: 39821398 Free PMC article.
-
Psychological Therapies Used for the Reduction of Habitual Cigarette Smoking Cigarette Consumption: A Systematic Review.Int J Environ Res Public Health. 2024 Jun 9;21(6):753. doi: 10.3390/ijerph21060753. Int J Environ Res Public Health. 2024. PMID: 38929001 Free PMC article.
References
-
- World Health Organization . Global report on trends in prevalence of tobacco smoking.2015. Available at: http://www.who.int/tobacco/publications/surveillance/reportontrendstobac... .
-
- Statistics on smoking . England: 2018. Available at: https://files.digital.nhs.uk/0C/95F481/stat‐smok‐eng‐2018‐rep.pdf .
-
- Bucchalter A. R., Acosta M. C., Evans S. E., Breland A. B., Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking‐related stimuli that are delivered by denicotinized cigarettes. Addiction 2015; 100: 550–559. - PubMed
-
- Public Health England . Health matters: Smoking and quitting in England. https://www.gov.uk/government/publications/health-matters-smoking-and-qu.... 2016.
-
- Ronckers E. T., Groot W., Ament A. J. Systematic review of economic evaluations of smoking cessation: standardizing the cost‐effectiveness. Med Decis Making 2005; 25: 437–448. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous