Printed PEDOT:PSS Trilayer: Mechanism Evaluation and Application in Energy Storage
- PMID: 31968612
- PMCID: PMC7013632
- DOI: 10.3390/ma13020491
Printed PEDOT:PSS Trilayer: Mechanism Evaluation and Application in Energy Storage
Abstract
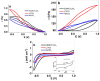
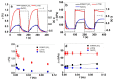
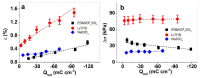
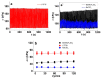
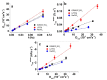
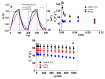
Combining ink-jet printing and one of the most stable electroactive materials, PEDOT:PSS (poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate)), is envisaged to pave the way for the mass production of soft electroactive materials. Despite its being a well-known electroactive material, widespread application of PEDOT:PSS also requires good understanding of its response. However, agreement on the interpretation of the material's activities, notably regarding actuation, is not unanimous. Our goal in this work is to study the behavior of trilayers with PEDOT:PSS electrodes printed on either side of a semi-interpenetrated polymer network membrane in propylene carbonate solutions of three different electrolytes, and to compare their electroactive, actuation, and energy storage behavior. The balance of apparent faradaic and non-faradaic processes in each case is discussed. The results show that the primarily cation-dominated response of the trilayers in the three electrolytes is actually remarkably different, with some rather uncommon outcomes. The different balance of the apparent charging mechanisms makes it possible to clearly select one electrolyte for potential actuation and another for energy storage application scenarios.
Keywords: PEDOT:PSS-IPN trilayer; energy storage; ink-jet printing; linear actuation; three different electrolytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Saetia K., Schnorr J.M., Mannarino M.M., Kim S.Y., Rutledge G.C., Swager T.M., Hammond P.T. Spray-layer-by-layer carbon nanotube/electrospun fiber electrodes for flexible chemiresistive sensor applications. Adv. Funct. Mater. 2014;24:492–502. doi: 10.1002/adfm.201302344. - DOI
-
- Afzal A., Abuilaiwi F.A., Habib A., Awais M., Waje S.B., Atieh M.A. Polypyrrole/carbon nanotube supercapacitorsTechnological advances and challenges. J. Power Sources. 2017;352:174–186. doi: 10.1016/j.jpowsour.2017.03.128. - DOI
-
- Zhou Y., Zhang F., Tvingstedt K., Barrau S., Li F., Tian W., Inganäs O. Investigation on polymer anode design for flexible polymer solar cells. Appl. Phys. Lett. 2008;92:92–94. doi: 10.1063/1.2945796. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

