Schistosomiasis vaccine development: update on human clinical trials
- PMID: 31969170
- PMCID: PMC6977295
- DOI: 10.1186/s12929-020-0621-y
Schistosomiasis vaccine development: update on human clinical trials
Abstract
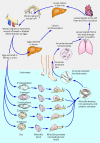
Schistosomiasis causes significant levels of morbidity and mortality in many geographical regions of the world. The disease is caused by infections with parasitic blood flukes known as schistosomes. The control of schistosomiasis over the last several decades has been centered on the mass drug administration (MDA) of praziquantel (PZQ), which is the only drug currently available for treatment. Despite the concerted efforts of MDA programs, the prevalence and transmission of schistosomiasis has remained largely unchecked due to the fact that PZQ is ineffective against juvenile schistosomes, does not prevent re-infection and the emergence of PZQ-resistant parasites. In addition, other measures such as the water, sanitation and hygiene programs and snail intermediate hosts control have had little to no impact. These drawbacks indicate that the current control strategies are severely inadequate at interrupting transmission and therefore, implementation of other control strategies are required. Ideally, an efficient vaccine is what is needed for long term protection thereby eliminating the current efforts of repeated mass drug administration. However, the general consensus in the field is that the integration of a viable vaccine with MDA and other control measures offer the best chance of achieving the goal of schistosomiasis elimination. This review focuses on the present status of schistosomiasis vaccine candidates in different phases of human clinical trials and provide some insight into future vaccine discovery and design.
Keywords: Clinical trials; Control strategies; Mass drug administration (MDA); Neglected tropical disease; Schistosomiasis; Schistosomiasis vaccine development.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

