Complementary Control over Habits and Behavioral Vigor by Phasic Activity in the Dorsolateral Striatum
- PMID: 31969469
- PMCID: PMC7055129
- DOI: 10.1523/JNEUROSCI.1313-19.2019
Complementary Control over Habits and Behavioral Vigor by Phasic Activity in the Dorsolateral Striatum
Abstract
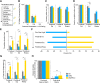
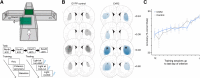
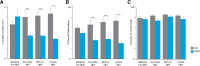
Despite clear evidence linking the basal ganglia to the control of outcome insensitivity (i.e., habit) and behavioral vigor (i.e., its behavioral speed/fluidity), it remains unclear whether or how these functions relate to one another. Here, using male Long-Evans rats in response-based and cue-based maze-running tasks, we demonstrate that phasic dorsolateral striatum (DLS) activity occurring at the onset of a learned behavior regulates how vigorous and habitual it is. In a response-based task, brief optogenetic excitation at the onset of runs decreased run duration and the occurrence of deliberative behaviors, whereas midrun stimulation carried little effect. Outcome devaluation showed these runs to be habitual. DLS inhibition at run start did not produce robust effects on behavior until after outcome devaluation. At that time, when the DLS was plausibly most critically required for performance (i.e., habitual), inhibition reduced performance vigor measures and caused a dramatic loss of habitual responding (i.e., animals quit the task). In a second cue-based "beacon" task requiring behavior initiation at the start of the run and again in the middle of the run, DLS excitation at both time points could improve the vigor of runs. Postdevaluation testing showed behavior on the beacon task to be habitual as well. This pattern of results suggests that one role for phasic DLS activity at behavior initiation is to promote the execution of the behavior in a vigorous and habitual fashion by a diverse set of measures.SIGNIFICANCE STATEMENT Our research expands the literature twofold. First, we find that features of a habitual behavior that are typically studied separately (i.e., maze response performance, deliberation movements, running vigor, and outcome insensitivity) are quite closely linked together. Second, efforts have been made to understand "what" the dorsolateral striatum (DLS) does for habitual behavior, and our research provides a key set of results showing "when" it is important (i.e., at behavior initiation). By showing such dramatic control over habits by DLS activity in a phasic time window, plausible real-world applications could involve more informed DLS perturbations to curb intractably problematic habits.
Keywords: basal ganglia; deliberation (VTE); dorsolateral striatum; goal-directed action; habit; optogenetics; outcome insensitivity; plus-maze; vigor.
Copyright © 2020 the authors.
Figures


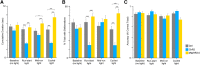

References
-
- Amaya KA, Smith KS (2018) Neurobiology of habit formation. Curr Opin Behav Sci 20:145–152. 10.1016/j.cobeha.2018.01.003 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
