Plant based production of myoglobin - a novel source of the muscle heme-protein
- PMID: 31969582
- PMCID: PMC6976567
- DOI: 10.1038/s41598-020-57565-y
Plant based production of myoglobin - a novel source of the muscle heme-protein
Abstract
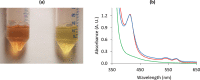
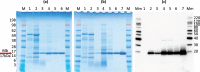
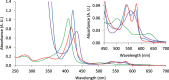
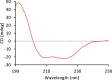
Myoglobin is a heme-protein in the muscle of vertebrates with important functions in the oxygenation of tissues and as a regulator in nitric oxide signaling. Myoglobin from many species is also an important nutritional source of bioavailable iron. In this study, we have successfully produced human myoglobin in the leaves of Nicotiana benthamiana by transient expression using a viral vector delivered by Agrobacterium tumefaciens. Analyses confirmed that heme was incorporated and the protein was functional, with observed properties consistent with those of native myoglobins. A relatively high degree of purity could be achieved with low cost methods. The results show the high potential of plants as a production platform for heme proteins, a group of proteins of interest for iron nutrition applications and possible future pharmaceutical development.
Conflict of interest statement
The authors declare no competing interests.
Figures


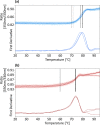
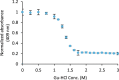
References
-
- Antonini, E. & Brunori, M. Hemoglobin and myoglobin in their reactions with ligands. (North Holland Pub. Co., 1971).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

