Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo
- PMID: 31969608
- PMCID: PMC6976711
- DOI: 10.1038/s41598-020-57666-8
Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo
Abstract
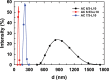
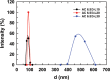
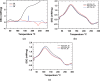
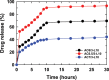
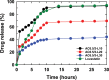
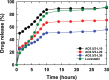
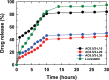
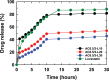
In this study, chitosan and alginate were selected to prepare alginate/chitosan nanoparticles to load the drug lovastatin by the ionic gelation method. The synthesized nanoparticles loaded with drug were characterized by Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), laser scattering and differential scanning calorimetry (DSC) methods. The FTIR spectrum of the alginate/chitosan/lovastatin nanoparticles showed that chitosan and alginate interacted with lovastatin through hydrogen bonding and dipolar-dipolar interactions between the C-O, C=O, and OH groups in lovastatin, the C-O, NH, and OH groups in chitosan and the C-O, C=O, and OH groups in alginate. The laser scattering results and SEM images indicated that the alginate/chitosan/lovastatin nanoparticles have a spherical shape with a particle size in the range of 50-80 nm. The DSC diagrams displayed that the melting temperature of the alginate/chitosan/lovastatin nanoparticles was higher than that of chitosan and lower than that of alginate. This result means that the alginate and chitosan interact together, so that the nanoparticles have a larger crystal degree when compared with alginate and chitosan individually. Investigations of the in vitro lovastatin release from the alginate/chitosan/lovastatin nanoparticles under different conditions, including different alginate/chitosan ratios, different solution pH values and different lovastatin contents, were carried out by ultraviolet-visible spectroscopy. The rate of drug release from the nanoparticles is proportional to the increase in the solution pH and inversely proportional to the content of the loaded lovastatin. The drug release process is divided into two stages: a rapid stage over the first 10 hr, then the release becomes gradual and stable. The Korsmeyer-Peppas model is most suitable for the lovastatin release process from the alginate/chitosan/lovastatin nanoparticles in the first stage, and then the drug release complies with other models depending on solution pH in the slow release stage. In addition, the toxicity of alginate/chitosan/lovastatin (abbreviated ACL) nanoparticles was sufficiently low in mice in the acute toxicity test. The LD50 of the drug was higher than 5000 mg/kg, while in the subchronic toxicity test with treatments of 100 mg/kg and 300 mg/kg ACL nanoparticles, there were no abnormal signs, mortality, or toxicity in general to the function or structure of the crucial organs. The results show that the ACL nanoparticles are safe in mice and that these composite nanoparticles might be useful as a new drug carrier.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Sandhya P, Kodali G, Fatima A. Formulation and in-vitro characterization of gastro retentive mucoadhesive tablets of Lovastatin. Int. J. Biol. Pharm. Res. 2014;5(1):71–77.
-
- Anilkumar JS, Harinath NM. Lovastatin loaded chitosan nanoparticles: Preparation, evaluation and in vitro release studies. J. Pharm. Technol. 2011;4(12):1869–1876.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

