Efficient whole-cell catalysis for 5-aminovalerate production from L-lysine by using engineered Escherichia coli with ethanol pretreatment
- PMID: 31969619
- PMCID: PMC6976619
- DOI: 10.1038/s41598-020-57752-x
Efficient whole-cell catalysis for 5-aminovalerate production from L-lysine by using engineered Escherichia coli with ethanol pretreatment
Abstract
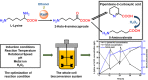
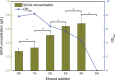
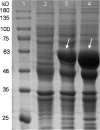
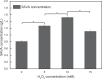
Microorganisms can utilize biomass to produce valuable chemicals, showing sustainable, renewable and economic advantages compared with traditional chemical synthesis. As a potential five-carbon platform polymer monomer, 5-aminovalerate has been widely used in industrial fields such as clothes and disposable goods. Here we establish an efficient whole-cell catalysis for 5-aminovalerate production with ethanol pretreatment. In this study, the metabolic pathway from L-lysine to 5-aminovalerate was constructed at the cellular level by introducing L-lysine α-oxidase. The newly produced H2O2 and added ethanol both are toxic to the cells, obviously inhibiting their growth. Here, a promising strategy of whole-cell catalysis with ethanol pretreatment is proposed, which greatly improves the yield of 5-aminovalerate. Subsequently, the effects of ethanol pretreatment, substrate concentration, reaction temperature, pH value, metal ion additions and hydrogen peroxide addition on the whole-cell biocatalytic efficiency were investigated. Using 100 g/L of L-lysine hydrochloride as raw material, 50.62 g/L of 5-aminovalerate could be excellently produced via fed-batch bioconversion with the yield of 0.84 mol/mol. The results show that a fast, environmentally friendly and efficient production of 5-aminovalerate was established after introducing the engineered whole-cell biocatalysts. This strategy, combined with ethanol pretreatment, can not only greatly enhance the yield of 5-aminovalerate but also be applied to the biosynthesis of other valuable chemicals.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

