Constructing protein polyhedra via orthogonal chemical interactions
- PMID: 31969701
- PMCID: PMC7007351
- DOI: 10.1038/s41586-019-1928-2
Constructing protein polyhedra via orthogonal chemical interactions
Abstract
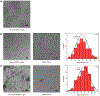
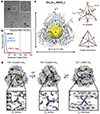
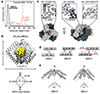
Many proteins exist naturally as symmetrical homooligomers or homopolymers1. The emergent structural and functional properties of such protein assemblies have inspired extensive efforts in biomolecular design2-5. As synthesized by ribosomes, proteins are inherently asymmetric. Thus, they must acquire multiple surface patches that selectively associate to generate the different symmetry elements needed to form higher-order architectures1,6-a daunting task for protein design. Here we address this problem using an inorganic chemical approach, whereby multiple modes of protein-protein interactions and symmetry are simultaneously achieved by selective, 'one-pot' coordination of soft and hard metal ions. We show that a monomeric protein (protomer) appropriately modified with biologically inspired hydroxamate groups and zinc-binding motifs assembles through concurrent Fe3+ and Zn2+ coordination into discrete dodecameric and hexameric cages. Our cages closely resemble natural polyhedral protein architectures7,8 and are, to our knowledge, unique among designed systems9-13 in that they possess tightly packed shells devoid of large apertures. At the same time, they can assemble and disassemble in response to diverse stimuli, owing to their heterobimetallic construction on minimal interprotein-bonding footprints. With stoichiometries ranging from [2 Fe:9 Zn:6 protomers] to [8 Fe:21 Zn:12 protomers], these protein cages represent some of the compositionally most complex protein assemblies-or inorganic coordination complexes-obtained by design.
Figures









References
-
- Marsh JA & Teichmann SA Structure, Dynamics, Assembly, and Evolution of Protein Complexes. Annu. Rev. Biochem 84, 551–575 (2015). - PubMed
-
- Bai Y, Luo Q & Liu J Protein self-assembly via supramolecular strategies. Chem. Soc. Rev 45, 2756–2767 (2016). - PubMed
-
- Churchfield LA & Tezcan FA Design and Construction of Functional Supramolecular Metalloprotein Assemblies. Acc. Chem. Res 52, 345–355 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

