Helicobacter pylori Dampens HLA-II Expression on Macrophages via the Up-Regulation of miRNAs Targeting CIITA
- PMID: 31969878
- PMCID: PMC6960189
- DOI: 10.3389/fimmu.2019.02923
Helicobacter pylori Dampens HLA-II Expression on Macrophages via the Up-Regulation of miRNAs Targeting CIITA
Abstract
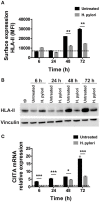
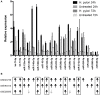
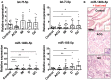
Macrophages have a major role in infectious and inflammatory diseases, and the available data suggest that Helicobacter pylori persistence can be explained in part by the failure of the bacterium to be killed by professional phagocytes. Macrophages are cells ready to kill the engulfed pathogen, through oxygen-dependent and -independent mechanisms; however, their killing potential can be further augmented by the intervention of T helper (Th) cells upon the specific recognition of human leukocyte antigen (HLA)-II-peptide complexes on the surface of the phagocytic cells. As it pertains to H. pylori, the bacterium is engulfed by macrophages, but it interferes with the phagosome maturation process leading to phagosomes with an altered degradative capacity, and to megasomes, wherein H. pylori resists killing. We recently showed that macrophages infected with H. pylori strongly reduce the expression of HLA-II molecules on the plasma membrane and this compromises the bacterial antigen presentation to Th lymphocytes. In this work, we demonstrate that H. pylori hampers HLA-II expression in macrophages, activated or non-activated by IFN-γ, by down-regulating the expression of the class II major histocompatibility complex transactivator (CIITA), the "master control factor" for the expression of HLA class II genes. We provided evidence that this effect relies on the up-regulation of let-7f-5p, let-7i-5p, miR-146b-5p, and -185-5p targeting CIITA. MiRNA expression analysis performed on biopsies from H. pylori-infected patients confirmed the up-regulation of let-7i-5p, miR-146b-5p, and -185-5p in gastritis, in pre-invasive lesions, and in gastric cancer. Taken together, our results suggest that specific miRNAs may be directly involved in the H. pylori infection persistence and may contribute to confer the risk of developing gastric neoplasia in infected patients.
Keywords: Helicobacter pylori; antigen presentation; gastric cancer; macrophages; miRNA.
Copyright © 2020 Codolo, Toffoletto, Chemello, Coletta, Soler Teixidor, Battaggia, Munari, Fassan, Cagnin and de Bernard.
Figures
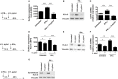



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

