Lack of Gut Secretory Immunoglobulin A in Memory B-Cell Dysfunction-Associated Disorders: A Possible Gut-Spleen Axis
- PMID: 31969880
- PMCID: PMC6960143
- DOI: 10.3389/fimmu.2019.02937
Lack of Gut Secretory Immunoglobulin A in Memory B-Cell Dysfunction-Associated Disorders: A Possible Gut-Spleen Axis
Abstract
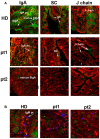
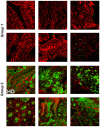
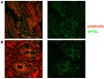
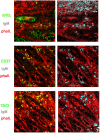
Background: B-1a B cells and gut secretory IgA (SIgA) are absent in asplenic mice. Human immunoglobulin M (IgM) memory B cells, which are functionally equivalent to mouse B-1a B cells, are reduced after splenectomy. Objective: To demonstrate whether IgM memory B cells are necessary for generating IgA-secreting plasma cells in the human gut. Methods: We studied intestinal SIgA in two disorders sharing the IgM memory B cell defect, namely asplenia, and common variable immune deficiency (CVID). Results: Splenectomy was associated with reduced circulating IgM memory B cells and disappearance of intestinal IgA-secreting plasma cells. CVID patients with reduced circulating IgM memory B cells had a reduced frequency of gut IgA+ plasma cells and a disrupted film of SIgA on epithelial cells. Toll-like receptor 9 (TLR9) and transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) induced IgM memory B cell differentiation into IgA+ plasma cells in vitro. In the human gut, TACI-expressing IgM memory B cells were localized under the epithelial cell layer where the TACI ligand a proliferation inducing ligand (APRIL) was extremely abundant. Conclusions: Circulating IgM memory B cell depletion was associated with a defect of intestinal IgA-secreting plasma cells in asplenia and CVID. The observation that IgM memory B cells have a distinctive role in mucosal protection suggests the existence of a functional gut-spleen axis.
Keywords: common variable immune deficiency; gut mucosal immunology; plasma cell; splenectomy; transmembrane activator and calcium-modulator and cyclophilin ligand interactor.
Copyright © 2020 Carsetti, Di Sabatino, Rosado, Cascioli, Piano Mortari, Milito, Grimsholm, Aranburu, Giorda, Tinozzi, Pulvirenti, Donato, Morini, Bagolan, Corazza and Quinti.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

