7T MRI Predicts Amelioration of Neurodegeneration in the Brain after AAV Gene Therapy
- PMID: 31970203
- PMCID: PMC6962699
- DOI: 10.1016/j.omtm.2019.11.023
7T MRI Predicts Amelioration of Neurodegeneration in the Brain after AAV Gene Therapy
Abstract
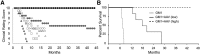
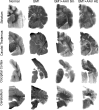
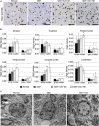
GM1 gangliosidosis (GM1) is a fatal neurodegenerative lysosomal storage disease that occurs most commonly in young children, with no effective treatment available. Long-term follow-up of GM1 cats treated by bilateral thalamic and deep cerebellar nuclei (DCN) injection of adeno-associated virus (AAV)-mediated gene therapy has increased lifespan to 8 years of age, compared with an untreated lifespan of ~8 months. Due to risks associated with cerebellar injection in humans, the lateral ventricle was tested as a replacement route to deliver an AAVrh8 vector expressing feline β-galactosidase (β-gal), the defective enzyme in GM1. Treatment via the thalamus and lateral ventricle corrected storage, myelination, astrogliosis, and neuronal morphology in areas where β-gal was effectively delivered. Oligodendrocyte number increased, but only in areas where myelination was corrected. Reduced AAV and β-gal distribution were noted in the cerebellum with subsequent increases in storage, demyelination, astrogliosis, and neuronal degeneration. These postmortem findings were correlated with endpoint MRI and magnetic resonance spectroscopy (MRS). Compared with the moderate dose with which most cats were treated, a higher AAV dose produced superior survival, currently 6.5 years. Thus, MRI and MRS can predict therapeutic efficacy of AAV gene therapy and non-invasively monitor cellular events within the GM1 brain.
Keywords: AAV; GM1; MRI; adeno-associated virus; biomarkers; gangliosidosis; gene therapy; lysosomal storage disease; spectroscopy.
© 2019.
Figures





References
-
- Regier D.S., Tifft C.J. GLB1-related disorders. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., editors. GeneReviews. University of Washington; 2013. - PubMed
-
- Suzuki Y., Sakuraba H., Oshima A. Beta-galactosidase deficiency (beta-galactosidosis): GM1 gangliosidosis and Morquio B disease. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. Seventh Edition. Volume 2. McGraw-Hill, Inc.; 1995. pp. 2785–2823.
-
- van der Voorn J.P., Kamphorst W., van der Knaap M.S., Powers J.M. The leukoencephalopathy of infantile GM1 gangliosidosis: oligodendrocytic loss and axonal dysfunction. Acta Neuropathol. 2004;107:539–545. - PubMed
-
- Imamura A., Miyajima H., Ito R., Orii K.O. Serial MR imaging and 1H-MR spectroscopy in monozygotic twins with Tay-Sachs disease. Neuropediatrics. 2008;39:259–263. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

