Hypomania and saccadic changes in Parkinson's disease: influence of D2 and D3 dopaminergic signalling
- PMID: 31970287
- PMCID: PMC6969176
- DOI: 10.1038/s41531-019-0107-3
Hypomania and saccadic changes in Parkinson's disease: influence of D2 and D3 dopaminergic signalling
Abstract
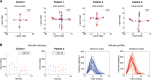
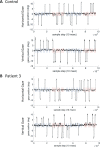
In order to understand the influence of two dopaminergic signalling pathways, TaqIA rs1800497 (influencing striatal D2 receptor density) and Ser9Gly rs6280 (influencing the striatal D3 dopamine-binding affinity), on saccade generation and psychiatric comorbidities in Parkinson's disease, this study aimed to investigate the association of saccadic performance in hypomanic or impulsive behaviour in parkinsonian patients; besides we questioned whether variants of D2 (A1+/A1-) and D3 (B1+/B1-) receptor polymorphism influence saccadic parameters differently, and if clinical parameters or brain connectivity changes modulate this association in the nigro-caudatal and nigro-collicular tract. Initially, patients and controls were compared regarding saccadic performance and differed in the parameter duration in memory-guided saccades (MGS) and visually guided saccades (VGS) trials (p < 0.0001) and in the MGS trial (p < 0.03). We were able to find associations between hypomanic behaviour (HPS) and saccade parameters (duration, latency, gain and amplitude) for both conditions [MGS (p = 0.036); VGS (p = 0.033)], but not for impulsive behaviour. For the A1 variant duration was significantly associated with HPS [VGS (p = 0.024); MGS (p = 0.033)]. In patients with the B1 variant, HPS scores were more consistently associated with duration [VGS (p = 0.005); MGS (p = 0.015), latency [VGS (p = 0.022)]] and amplitude [MGS (p = 0.006); VGS (p = 0.005)]. The mediation analysis only revealed a significant indirect effect for amplitude in the MGS modality for the variable UPDRS-ON (p < 0.05). All other clinical scales and brain connectivity parameters were not associated with behavioural traits. Collectively, our findings stress the role of striatal D2 and D3 signalling mechanisms in saccade generation and suggest that saccadic performance is associated with the clinical psychiatric state in Parkinson's disease.
Keywords: Clinical genetics; Oculomotor system; Parkinson's disease.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

