Acquisition of Antibiotic-Resistant Gram-negative Bacteria in the Benefits of Universal Glove and Gown (BUGG) Cluster Randomized Trial
- PMID: 31970393
- PMCID: PMC7850534
- DOI: 10.1093/cid/ciaa071
Acquisition of Antibiotic-Resistant Gram-negative Bacteria in the Benefits of Universal Glove and Gown (BUGG) Cluster Randomized Trial
Abstract
Background: The Benefits of Universal Glove and Gown (BUGG) cluster randomized trial found varying effects on methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus and no increase in adverse events. The aim of this study was to assess whether the intervention decreases the acquisition of antibiotic-resistant gram-negative bacteria.
Methods: This was a secondary analysis of a randomized trial in 20 hospital intensive care units. The intervention consisted of healthcare workers wearing gloves and gowns when entering any patient room compared to standard care. The primary composite outcome was acquisition of any antibiotic-resistant gram-negative bacteria based on surveillance cultures.
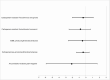
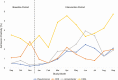
Results: A total of 40 492 admission and discharge perianal swabs from 20 246 individual patient admissions were included in the primary outcome. For the primary outcome of acquisition of any antibiotic-resistant gram-negative bacteria, the intervention had a rate ratio (RR) of 0.90 (95% confidence interval [CI], .71-1.12; P = .34). Effects on the secondary outcomes of individual bacteria acquisition were as follows: carbapenem-resistant Enterobacteriaceae (RR, 0.86 [95% CI, .60-1.24; P = .43), carbapenem-resistant Acinetobacter (RR, 0.81 [95% CI, .52-1.27; P = .36), carbapenem-resistant Pseudomonas (RR, 0.88 [95% CI, .55-1.42]; P = .62), and extended-spectrum β-lactamase-producing bacteria (RR, 0.94 [95% CI, .71-1.24]; P = .67).
Conclusions: Universal glove and gown use in the intensive care unit was associated with a non-statistically significant decrease in acquisition of antibiotic-resistant gram-negative bacteria. Individual hospitals should consider the intervention based on the importance of these organisms at their hospital, effect sizes, CIs, and cost of instituting the intervention.
Clinical trials registration: NCT01318213.
Keywords: antibiotic resistance; barrier precautions; contact precautions.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173:2039–46. - PubMed
-
- Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG; Antimicrobial Availability Task Force of the Infectious Diseases Society of America Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006; 42:657–68. - PubMed
-
- Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. - PMC - PubMed
-
- World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed Available at: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-ba.... Accessed 19 March 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

