The Alopecia Areata Investigator Global Assessment scale: a measure for evaluating clinically meaningful success in clinical trials
- PMID: 31970750
- PMCID: PMC7586961
- DOI: 10.1111/bjd.18883
The Alopecia Areata Investigator Global Assessment scale: a measure for evaluating clinically meaningful success in clinical trials
Abstract
Background: Content-valid and clinically meaningful instruments are required to evaluate outcomes of therapeutic interventions in alopecia areata (AA).
Objectives: To develop an Investigator's Global Assessment (IGA) to interpret treatment response in AA treatment studies.
Methods: Qualitative interviews were conducted in the USA with expert dermatologists and with patients with AA who had experienced ≥ 50% scalp-hair loss. Thematic data analysis identified critical outcomes and evaluated the content validity of the new IGA.
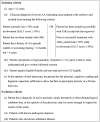
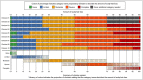
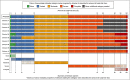
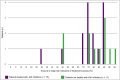
Results: Expert clinicians (n = 10) judged AA treatment success by the amount of scalp-hair growth (median 80% scalp hair). Adult (n = 25) and adolescent (n = 5) patients participated. Scalp-hair loss was the most bothersome AA sign/symptom for most patients. Perceived treatment success - short of 100% scalp hair - was the presence of ~ 70-90% scalp hair (median 80%). Using additional clinician and patient insights, the Alopecia Areata Investigator Global Assessment (AA-IGA™) was developed. This clinician-reported outcome assessment is an ordinal, static measure comprising five severity categories of scalp-hair loss. Nearly all clinicians and patients in this study agreed that, for patients with ≥ 50% scalp-hair loss, successful treatment would be hair regrowth resulting in ≤ 20% scalp-hair loss.
Conclusions: We recommend using the Severity of Alopecia Tool to assess the extent (0-100%) of scalp-hair loss. The AA-IGA is a robust ordinal measure providing distinct and clinically meaningful gradations of scalp-hair loss that reflects patients' and expert clinicians' perspectives and treatment expectations. What is already known about this topic? The Severity of Alopecia Tool is widely used to assess the extent of scalp-hair loss in patients with alopecia areata. Guidelines define treatment success as a 50% improvement in scalp hair, and clinical trials have used dynamic thresholds of 50% and 90%. However, there is no clinical consensus on these endpoints, and patient perspectives on treatment success are unknown. What does this study add? Through qualitative interviews with 10 expert dermatologists and 30 patients with alopecia areata who had experienced ≥ 50% scalp-hair loss, we developed the Alopecia Areata Investigator Global Assessment (AA-IGA™) to measure five clinically meaningful gradations of alopecia areata scalp-hair loss that reflects patients' and clinicians' perspectives and expectations of treatment success in alopecia areata treatment studies. What are the clinical implications of this work? The AA-IGA is a robust ordinal measure that can inform clinical evaluation of alopecia areata treatment outcomes. The AA-IGA can be used to determine clinically meaningful treatment success for alopecia areata, with success defined by patients and clinicians as reaching ≤ 20% scalp-hair loss. Linked Comment: Blome. Br J Dermatol 2020; 183:609.
© 2020 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures

Comment in
-
Percentage of scalp-hair loss as a primary endpoint in alopecia areata trials.Br J Dermatol. 2020 Oct;183(4):609. doi: 10.1111/bjd.19045. Epub 2020 Apr 27. Br J Dermatol. 2020. PMID: 32338381 No abstract available.
References
-
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health . Guidance for Industry Patient‐Reported Outcome Measures: Use in Medical Product Development To Support Labeling Claims. Food and Drug Administration, 2009. Available at: http://www.fda.gov/media/77832/download (last accessed 2 February 2020). - PubMed
-
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Patient‐Focused Drug Development: Collecting Comprehensive and Representative Input. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Draft Guidance. Food and Drug Administration, 2018. Available at: http://www.fda.gov/media/113653/download (last accessed 2 February 2020).
-
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research . Qualification Process for Drug Development Tools: Guidance for Industry and FDA Staff. Draft Guidance. Food and Drug Administration, 2019. Available at: http://www.fda.gov/media/133511/download (last accessed 2 February 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

