Stereoselective Synthesis of Baulamycin A
- PMID: 31970985
- PMCID: PMC7271067
- DOI: 10.1021/acs.joc.9b03443
Stereoselective Synthesis of Baulamycin A
Abstract
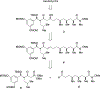
New structural classes of antibiotics are rare, structurally novel broad-spectrum antibiotics exceptionally so. The recently discovered baulamycins constitute a remarkable example of these highly prized compounds and, as such, have attracted considerable attention in the form of both synthetic efforts and biological studies. For the first time, we report a gram-scale preparation of the common carbon framework of the baulamycin family, as well as the total synthesis of its most potent member, baulamycin A. Our approach employs highly stereoselective, catalyst-controlled asymmetric conjugate additions to thioesters to set key stereocenters, as well as the first reported use of "dry ozonolysis" to reveal a masked carboxylic acid in the total synthesis of a natural product.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


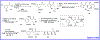
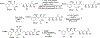
References
-
- Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017; World Health Organization: Geneva, 2017.
-
- Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention, 2013.
-
- Tacconelli E; Pezzani MD Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis 2019, 19, 4–6. - PubMed
-
- Hawkey PM; Jones AM The changing epidemiology of resistance. J. Antimicrob. Chemother 2009, 64, i3–i10. - PubMed
-
- Tripathi A; Schofield MM; Chlipala GE; Schultz PJ; Yim I; Newmister SA; Nusca TD; Scaglione JB; Hanna PC; Tamayo-Castillo G; Sherman DH Baulamycins A and B, broad-spectrum antibiotics identified as inhibitors of siderophore biosyn-thesis in Staphylococcus aureus and Bacillus anthracis. J. Am. Chem. Soc 2014, 136, 1579–1586. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

