Regulatory role of SphK1 in TLR7/9-dependent type I interferon response and autoimmunity
- PMID: 31971297
- PMCID: PMC7559288
- DOI: 10.1096/fj.201902847R
Regulatory role of SphK1 in TLR7/9-dependent type I interferon response and autoimmunity
Abstract
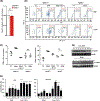
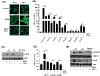
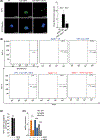
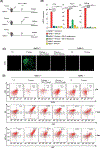
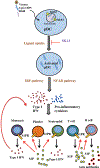
Plasmacytoid dendritic cells (pDCs) express Toll like receptors (TLRs) that modulate the immune response by production of type I interferons. Here, we report that sphingosine kinase 1 (SphK1) which produces the bioactive sphingolipid metabolite, sphingosine 1-phosphate (S1P), plays a critical role in the pDC functions and interferon production. Although dispensable for the pDC development, SphK1 is essential for the pDC activation and production of type I IFN and pro-inflammatory cytokines stimulated by TLR7/9 ligands. SphK1 interacts with TLRs and specific inhibition or deletion of SphK1 in pDCs mitigates uptake of CpG oligonucleotide ligands by TLR9 ligand. In the pristane-induced murine lupus model, pharmacological inhibition of SphK1 or its genetic deletion markedly decreased the IFN signature, pDC activation, and glomerulonephritis. Moreover, increases in the SphK1 expression and S1P levels were observed in human lupus patients. Taken together, our results indicate a pivotal regulatory role for the SphK1/S1P axis in maintaining the balance between immunosurveillance and immunopathology and suggest that specific SphK1 inhibitors might be a new therapeutic avenue for the treatment of type I IFN-linked autoimmune disorders.
Keywords: auto-immunity; interferon; plasmacytoid dendritic cells; sphingosine 1-Phosphate; sphingosine kinase; systemic lupus erythematosus.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST
Dr Spiegel is a co-inventor on patent number US 8,372,888 B2 titled Sphingosine kinase type 1 inhibitors, compositions, and processes for using same. The other authors declare no competing interests.
Figures



References
-
- Alculumbre S, Raieli S, Hoffmann C, Chelbi R, Danlos FX, Soumelis V. Plasmacytoid pre-dendritic cells (pDC): from molecular pathways to function and disease association. Semin Cell Dev Biol. 2019;86:24–35. - PubMed
-
- Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. - PubMed
-
- Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. - PubMed
-
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

