Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020
- PMID: 31971931
- PMCID: PMC7367698
- DOI: 10.15585/mmwr.mm6903e2
Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020
Abstract
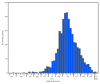
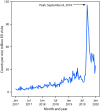
Since August 2019, CDC, the Food and Drug Administration (FDA), state and local health departments, and public health and clinical stakeholders have been investigating a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) (1). This report updates patient demographic characteristics, self-reported substance use, and hospitalization dates for EVALI patients reported to CDC by states, as well as the distribution of emergency department (ED) visits related to e-cigarette, or vaping, products analyzed through the National Syndromic Surveillance Program (NSSP). As of January 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC. Median patient age was 24 years, and 66% were male. Overall, 82% of EVALI patients reported using any tetrahydrocannabinol (THC)-containing e-cigarette, or vaping, product (including 33% with exclusive THC-containing product use), and 57% of EVALI patients reported using any nicotine-containing product (including 14% with exclusive nicotine-containing product use). Syndromic surveillance indicates that ED visits related to e-cigarette, or vaping, products continue to decline after sharply increasing in August 2019 and peaking in September 2019. Clinicians and public health practitioners should remain vigilant for new EVALI cases. CDC recommends that persons not use THC-containing e-cigarette, or vaping, products, especially those acquired from informal sources such as friends, family members, or from in-person or online dealers. Vitamin E acetate is strongly linked to the EVALI outbreak and should not be added to any e-cigarette, or vaping, products (2). However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC-containing products, in some reported EVALI cases.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Moritz ED, Zapata LB, Lekiachvili A, et al.; Lung Injury Response Epidemiology/Surveillance Group. Update: characteristics of patients in a national outbreak of e-cigarette, or vaping, product use–associated lung injuries—United States, October 2019. MMWR Morb Mortal Wkly Rep 2019;68:985–9. https://www.cdc.gov/mmwr/volumes/68/wr/mm6843e1.htm?s_cid=mm6843e1_w - PMC - PubMed
-
- CDC. National Syndromic Surveillance Program (NSSP): what is syndromic surveillance? Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/nssp/overview.html
-
- Hartnett KP, Kite-Powell A, Patel MT, et al. Syndromic surveillance for e-cigarette, or vaping, product use–associated lung injury. N Engl J Med 2019. https://www.nejm.org/doi/10.1056/NEJMsr1915313 - DOI - PMC - PubMed
-
- Ellington S, Salvatore PP, Ko J, et al.; Lung Injury Response Epidemiology/Surveillance Task Force. Update: product, substance-use, and demographic characteristics of hospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep 2020;68. 10.15585/mmwr.mm6902e210.15585/mmwr.mm685152e1 - DOI - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

