The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis
- PMID: 31971984
- PMCID: PMC6999914
- DOI: 10.1371/journal.ppat.1008236
The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis
Abstract
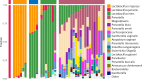
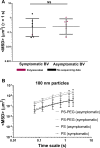
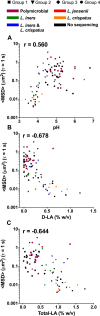
Bacterial vaginosis (BV), a condition in which the vaginal microbiota consists of community of obligate and facultative anaerobes rather than dominated by a single species of Lactobacillus, affects ~30% of women in the US. Women with BV are at 60% increased risk for HIV acquisition and are 3-times more likely to transmit HIV to an uninfected partner. As cervicovaginal mucus (CVM) is the first line of defense against mucosal pathogens and the home of the resident vaginal microbiota, we hypothesized the barrier function of CVM to HIV may be diminished in BV. Here, we characterized CVM properties including pH, lactic acid content, and Nugent score to correlate with the microbiota community composition, which was confirmed by 16S rDNA sequencing on a subset of samples. We then quantified the mobility of fluorescently-labeled HIV virions and nanoparticles to characterize the structural and adhesive barrier properties of CVM. Our analyses included women with Nugent scores categorized as intermediate (4-6) and BV (7-10), women that were either symptomatic or asymptomatic, and a small group of women before and after antibiotic treatment for symptomatic BV. Overall, we found that HIV virions had significantly increased mobility in CVM from women with BV compared to CVM from women with Lactobacillus crispatus-dominant microbiota, regardless of whether symptoms were present. We confirmed using nanoparticles and scanning electron microscopy that the impaired barrier function was due to reduced adhesive barrier properties without an obvious degradation of the physical CVM pore structure. We further confirmed a similar increase in HIV mobility in CVM from women with Lactobacillus iners-dominant microbiota, the species most associated with transitions to BV and that persists after antibiotic treatment for BV. Our findings advance the understanding of the protective role of mucus and highlight the interplay between vaginal microbiota and the innate barrier function mucus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sexually transmitted diseases. 2007;34(11):864–9. Epub 2007/07/11. 10.1097/OLQ.0b013e318074e565 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

