A method to study protein biomarkers in saliva using an automated capillary nano-immunoassay platform (Wes™)
- PMID: 31972214
- PMCID: PMC11416074
- DOI: 10.1016/j.jim.2020.112749
A method to study protein biomarkers in saliva using an automated capillary nano-immunoassay platform (Wes™)
Abstract
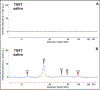
Traditional immunoprobing techniques like Western blot continue to play a crucial role in the discovery and validation of biomarkers. This technique suffers from several limitations that affect reproducibility and feasibility for large-scale studies. Modern immunoprobing techniques have addressed several of these limitations. Here we contrast the use of Western blot and an automated capillary nano-immunoassay (CNIA), Wes™. We provide evidence highlighting the methodological advantages of Wes™ over Western blot in the validation of a novel biomarker, Calcium-binding protein and spermatid-associated 1 (hCABS1). While Wes™ offers a faster, more consistent approach with lower requirements for sample and antibody volumes, variations in expected molecular weights and computational algorithms used to analyze the data must receive careful consideration and assessment. Our data suggests that CNIA approaches are likely to positively impact biomarker discovery and validation.
Keywords: Biomarker(s); CABS1; Capillary nano-immunoassay; Immunoprobing; Saliva; Stress; Western blot; Wes™.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures


References
-
- Calvel P, Kervarrec C, Lavigne R, Vallet-Erdtmann V, Guerrois M, Rolland AD, Chalmel F, Jégou B, Pineau C, 2009. CLPH, a novel casein kinase 2-phosphorylated disordered protein, is specifically associated with postmeiotic germ cells in rat spermatogenesis. J. Proteome Res. 10.1021/pr900082m - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

