Redox homeostasis, oxidative stress and mitophagy
- PMID: 31972372
- PMCID: PMC7062406
- DOI: 10.1016/j.mito.2020.01.002
Redox homeostasis, oxidative stress and mitophagy
Abstract
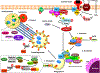
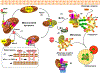
Autophagy is a ubiquitous homeostatic mechanism for the degradation or turnover of cellular components. Degradation of mitochondria via autophagy (mitophagy) is involved in a number of physiological processes including cellular homeostasis, differentiation and aging. Upon stress or injury, mitophagy prevents the accumulation of damaged mitochondria and the increased steady state levels of reactive oxygen species leading to oxidative stress and cell death. A number of human diseases, particularly neurodegenerative disorders, have been linked to the dysregulation of mitophagy. In this mini-review, we aimed to review the molecular mechanisms involved in the regulation of mitophagy and their relationship with redox signaling and oxidative stress.
Keywords: Autophagy; Fission; Fusion; Mitochondrial dynamics.
Copyright © 2020 Elsevier B.V. and Mitochondria Research Society. All rights reserved.
Figures
Similar articles
-
Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner.Biochim Biophys Acta. 2012 Dec;1823(12):2297-310. doi: 10.1016/j.bbamcr.2012.08.007. Epub 2012 Aug 16. Biochim Biophys Acta. 2012. PMID: 22917578
-
New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases.Biomed Pharmacother. 2024 Sep;178:117084. doi: 10.1016/j.biopha.2024.117084. Epub 2024 Aug 1. Biomed Pharmacother. 2024. PMID: 39088967 Review.
-
Oxidative stress-induced autophagy in plants: the role of mitochondria.Plant Physiol Biochem. 2012 Oct;59:11-9. doi: 10.1016/j.plaphy.2012.02.013. Epub 2012 Feb 16. Plant Physiol Biochem. 2012. PMID: 22386760 Review.
-
Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling.Biochem J. 2012 Jan 15;441(2):523-40. doi: 10.1042/BJ20111451. Biochem J. 2012. PMID: 22187934 Free PMC article. Review.
-
Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications.Redox Biol. 2017 Apr;11:637-645. doi: 10.1016/j.redox.2017.01.013. Epub 2017 Jan 16. Redox Biol. 2017. PMID: 28131082 Free PMC article. Review.
Cited by
-
Construction and evaluation of Alzheimer's disease diagnostic prediction model based on genes involved in mitophagy.Front Aging Neurosci. 2023 Mar 23;15:1146660. doi: 10.3389/fnagi.2023.1146660. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37032823 Free PMC article.
-
Endogenous and Exogenous Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells: An Updated Antioxidant Perspective.Int J Mol Sci. 2023 Jun 28;24(13):10776. doi: 10.3390/ijms241310776. Int J Mol Sci. 2023. PMID: 37445953 Free PMC article. Review.
-
Loss of PTEN-Induced Kinase 1 Regulates Oncogenic Ras-Driven Tumor Growth By Inhibiting Mitochondrial Fission.Front Oncol. 2022 May 5;12:893396. doi: 10.3389/fonc.2022.893396. eCollection 2022. Front Oncol. 2022. PMID: 35600352 Free PMC article.
-
Oxidative Stress in Cutaneous Lichen Planus-A Narrative Review.J Clin Med. 2021 Jun 18;10(12):2692. doi: 10.3390/jcm10122692. J Clin Med. 2021. PMID: 34207416 Free PMC article. Review.
-
Molecular pathways in experimental glaucoma models.Front Neurosci. 2024 Mar 18;18:1363170. doi: 10.3389/fnins.2024.1363170. eCollection 2024. Front Neurosci. 2024. PMID: 38562304 Free PMC article. Review.
References
-
- Aguiar AS Jr., Tristao FS, Amar M, Chevarin C, Lanfumey L, Mongeau R, Corti O, Prediger RD, Raisman-Vozari R, 2013. Parkin-knockout mice did not display increased vulnerability to intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Neurotox Res 24, 280–287. - PubMed
-
- Amaya C, Fader CM, Colombo MI, 2015. Autophagy and proteins involved in vesicular trafficking. FEBS Lett 589, 3343–3353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

