Advances in studying protein disorder with solid-state NMR
- PMID: 31972419
- PMCID: PMC7202078
- DOI: 10.1016/j.ssnmr.2020.101643
Advances in studying protein disorder with solid-state NMR
Abstract
Solution NMR is a key tool to study intrinsically disordered proteins (IDPs), whose importance for biological function is widely accepted. However, disordered proteins are not limited to solution and are also found in non-soluble systems such as fibrils and membrane proteins. In this Trends article, I will discuss how solid-state NMR can be used to study disorder in non-soluble proteins. Techniques based on dipolar couplings can study static protein disorder which either occurs naturally as e.g. in spider silk or can be induced by freeze trapping IDPs or unfolded proteins. In this case, structural ensembles are directly reflected by a static distribution of dihedral angels that can be determined by the distribution of chemical shifts or other methods. Techniques based on J-couplings can detect dynamic protein disorder under MAS. In this case, only average chemical shifts are measured but disorder can be characterized with a variety of data including secondary chemical shifts, relaxation rates, paramagnetic relaxation enhancements, or residual dipolar couplings. I describe both technical aspects and examples of solid-state NMR on protein disorder and end the article with a discussion of challenges and opportunities of this emerging field.
Keywords: Frozen solution; Intrinsically disordered proteins; Protein dynamics; Protein folding; Scalar coupling based methods; Solid-state NMR.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

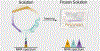



References
-
- Uversky VN, Chapter Four - Intrinsic Disorder, Protein–Protein Interactions, and Disease, in: Donev R (Ed.), Advances in Protein Chemistry and Structural Biology, Vol. 110 of Protein-Protein Interactions in Human Disease, Part A, Academic Press, 2018, pp. 85–121. doi:10.1016/bs.apcsb.2017.06.005. - DOI - PubMed
-
- Turoverov KK, Kuznetsova IM, Fonin AV, Darling AL, Zaslavsky BY, Uversky VN, Stochasticity of Biological Soft Matter: Emerging Concepts in Intrinsically Disordered Proteins and Biological Phase Separation, Trends in Biochemical Sciences 44 (8) (2019) 716–728. doi:10.1016/j.tibs.2019.03.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

