Novel roles of phosphoinositides in signaling, lipid transport, and disease
- PMID: 31972475
- PMCID: PMC7247936
- DOI: 10.1016/j.ceb.2019.12.007
Novel roles of phosphoinositides in signaling, lipid transport, and disease
Abstract
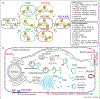
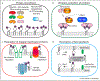
Phosphoinositides (PPIns) are lipid signaling molecules that act as master regulators of cellular signaling. Recent studies have revealed novel roles of PPIns in myriad cellular processes and multiple human diseases mediated by misregulation of PPIn signaling. This review will present a timely summary of recent discoveries in PPIn biology, specifically their role in regulating unexpected signaling pathways, modification of signaling outcomes downstream of integral membrane proteins, and novel roles in lipid transport. This has revealed new roles of PPIns in regulating membrane trafficking, immunity, cell polarity, and response to extracellular signals. A specific focus will be on novel opportunities to target PPIn metabolism for treatment of human diseases, including cancer, pathogen infection, developmental disorders, and immune disorders.
Keywords: Flippases; GPCR; Ion channels; Lipid kinases; Lipid signaling; Lipid transfer proteins; Membrane contact sites; Membrane trafficking; PI3P; PI4KB; PI4P; PIK3CA; PIP2; PIP3; Phosphatidylinositol; Phosphoinositide kinases; Phosphoinositides.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures


References
-
-
Goncalves MD, Cantley LC: Phosphatidylinositol 3-Kinase, Growth Disorders, and Cancer. N. Engl. J. Med. 2018, 379:2052–2062.
* An excellent review on the involvement of PI3Ks in pathological conditions, and novel therapeutic strategies to target them in disease,
-
-
- Burke JE: Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell 2018, 71:653–673. - PubMed
-
- Ketel K, Krauss M, Nicot A-S, Puchkov D, Wieffer M, Müller R, Subramanian D, Schultz C, Laporte J, Haucke V: A phosphoinositide conversion mechanism for exit from endosomes. Nature 2016, 529:408–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

