Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility
- PMID: 31972508
- PMCID: PMC6974790
- DOI: 10.1016/j.redox.2020.101431
Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility
Abstract
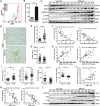
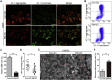
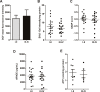
Endometriosis an important cause of female infertility and seriously impact physical and psychological health of patients. Endometriosis is now considered to be a public health problem that deserves in-depth investigation, especially the etiopathogenesis of endometriosis-associated infertility. We aimed to illuminate the etiopathogenesis of endometriosis-associated infertility that involve excessive oxidative stress (OS) induced pathological changes of ovary cumulus granulosa cell (GCs). Senescence-associated β-galactosidase (SA β-gal) activity in GCs from endometriosis patients, soluble isoform of advanced glycation end products receptor (sRAGE) expression in follicular fluid from endometriosis patients and differentially expressed senescence-associated secretory phenotype factors (IL-1β, MMP-9, KGF and FGF basic protein) are all useful indexes to evaluate oocyte retrieval number and mature oocyte number. RNA-sequencing and bioinformatics analysis indicated senescent phenotype of endometriosis GCs and aggravated endoplasmic reticulum (ER) stress in endometriosis GCs. Targeting ER stress significantly alleviated OS-induced GCs senescence as well as mitochondrial membrane potential (MMP) and adenosine triphosphate (ATP) reduction in GCs. Moreover, melatonin administration rescued OS-enhanced ER stress, cellular senescence, and MMP and ATP abnormities of endometriosis GCs in vitro and in vivo. In conclusion, our results indicated excessive reactive oxygen species induces senescence of endometriosis GCs via arouse ER stress, which finally contributes to endometriosis-associated infertility, and melatonin may represent a novel adjuvant therapy strategy for endometriosis-associated infertility.
Keywords: Cumulus granulosa cell; Endometriosis; Endoplasmic reticulum stress; Infertility; Oxidative stress; Senescence.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflict of interest.
Figures






References
-
- Vercellini P., Vigano P., Somigliana E., Fedele L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 2014;10(5):261–275. - PubMed
-
- Dumesic D.A., Meldrum D.R., Katz-Jaffe M.G., Krisher R.L., Schoolcraft W.B. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015;103(2):303–316. - PubMed
-
- Da Broi M.G., de Albuquerque F.O., de Andrade A.Z., Cardoso R.L., Jordao Junior A.A., Navarro P.A. Increased concentration of 8-hydroxy-2'-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016;366(1):231–242. - PubMed
-
- Da Broi M.G., Jordao A.A., Jr., Ferriani R.A., Navarro P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018;85(2):128–136. - PubMed
-
- Albertini D.F., Combelles C.M., Benecchi E., Carabatsos M.J. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

