Intravenous Ceftriaxone Versus Multiple Dosing Regimes of Intravenous Anti-Staphylococcal Antibiotics for Methicillin-Susceptible Staphylococcus aureus (MSSA): A Systematic Review
- PMID: 31972972
- PMCID: PMC7169384
- DOI: 10.3390/antibiotics9020039
Intravenous Ceftriaxone Versus Multiple Dosing Regimes of Intravenous Anti-Staphylococcal Antibiotics for Methicillin-Susceptible Staphylococcus aureus (MSSA): A Systematic Review
Abstract
Background: Methicillin-susceptible Staphylococcus aureus (MSSA) is a common pathogen associated with a range of clinically important infections. MSSA can cause deep-seated infections requiring prolonged courses of intravenous antibiotic therapy to achieve effective resolution. The move toward ambulatory or outpatient delivery of parenteral antibiotics has led to an increase in the use of ceftriaxone as a pragmatic first choice given its advantageous single daily dosing schedule.
Objective: To compare the efficacy of once daily ceftriaxone in the treatment of infections due to confirmed or suspected MSSA to multiple dosing regimes of anti-staphylococcal antibiotics.
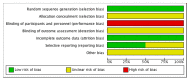
Methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Global Health, PubMed, EMBASE and CINAHL for randomised controlled trials as well as prospective and retrospective cohort studies that compared ceftriaxone to any multiple dosing regime of anti-staphylococcal antibiotics. Outcome measures were the proportion of patients with a resolution of infection based on time after initiation of therapy, adverse reactions, recurrence and duration of hospital admission.
Results: We included two randomized controlled trials, one prospective observational study and three retrospective cohort studies (643 participants; 246 children, 397 adults). There was no difference in time to resolution of symptoms. The number of adverse reactions, recurrence of bacteraemia and duration of hospital stay were not significantly different between ceftriaxone and other anti-staphylococcal antibiotics.
Conclusions: Based on a small number of low-quality studies, ceftriaxone is as effective as multiple dosing regimes for the treatment of infections due MSSA. An appropriately powered randomized trial is required to demonstrate equivalence and cost effectiveness.
Keywords: anti-staphlococcal antibiotics; intravenous ceftriaxone; mssa; multiple dosing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ceftriaxone versus antistaphylococcal antibiotics for definitive treatment of methicillin-susceptible Staphylococcus aureus infections: a systematic review and meta-analysis.Int J Antimicrob Agents. 2022 Jan;59(1):106486. doi: 10.1016/j.ijantimicag.2021.106486. Epub 2021 Nov 26. Int J Antimicrob Agents. 2022. PMID: 34839007
-
Safety and Efficacy of Ceftriaxone in the Treatment of Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections: A Noninferiority Retrospective Cohort Study.Ann Pharmacother. 2023 Apr;57(4):425-431. doi: 10.1177/10600280221115460. Epub 2022 Aug 9. Ann Pharmacother. 2023. PMID: 35942602
-
Outcomes of ceftriaxone use compared to standard of therapy in methicillin susceptible staphylococcal aureus (MSSA) bloodstream infections.Int J Clin Pharm. 2014 Dec;36(6):1282-9. doi: 10.1007/s11096-014-9999-5. Epub 2014 Sep 4. Int J Clin Pharm. 2014. PMID: 25186790
-
Ceftriaxone for the Treatment of Methicillin-susceptible Staphylococcus aureus Bacteremia: A Case Series.J Pharmacol Pharmacother. 2017 Jul-Sep;8(3):140-144. doi: 10.4103/jpp.JPP_5_17. J Pharmacol Pharmacother. 2017. PMID: 29081626 Free PMC article.
-
Clinical cure with ceftriaxone versus ceftaroline or ceftobiprole in the treatment of staphylococcal pneumonia: a systematic review and meta-analysis.Int J Antimicrob Agents. 2019 Aug;54(2):149-153. doi: 10.1016/j.ijantimicag.2019.05.023. Epub 2019 Jun 4. Int J Antimicrob Agents. 2019. PMID: 31173864
Cited by
-
Rational Use of Ceftriaxone in Necrotizing Fasciitis and Mortality Associated with Bloodstream Infection and Hemorrhagic Bullous Lesions.Antibiotics (Basel). 2022 Oct 22;11(11):1454. doi: 10.3390/antibiotics11111454. Antibiotics (Basel). 2022. PMID: 36358109 Free PMC article.
-
Synergistic Antimicrobial Activity of Ceftriaxone and Polyalthia longifolia Methanol (MEPL) Leaf Extract against Methicillin-Resistant Staphylococcus aureus and Modulation of mecA Gene Presence.Antibiotics (Basel). 2023 Feb 27;12(3):477. doi: 10.3390/antibiotics12030477. Antibiotics (Basel). 2023. PMID: 36978344 Free PMC article.
-
Coinfections with Bacteria, Fungi, and Respiratory Viruses in Patients with SARS-CoV-2: A Systematic Review and Meta-Analysis.Pathogens. 2021 Jun 25;10(7):809. doi: 10.3390/pathogens10070809. Pathogens. 2021. PMID: 34202114 Free PMC article. Review.
-
Effectiveness and Safety of Ceftriaxone Compared to Standard of Care for Treatment of Bloodstream Infections Due to Methicillin-Susceptible Staphylococcus aureus: A Systematic Review and Meta-Analysis.Antibiotics (Basel). 2022 Mar 10;11(3):375. doi: 10.3390/antibiotics11030375. Antibiotics (Basel). 2022. PMID: 35326838 Free PMC article.
References
-
- McDanel J.S., Perencevich E.N., Diekema D.J., Herwaldt L.A., Smith T.C., Chrischilles E.A., Dawson J.D., Jiang L., Goto M., Schweizer M.L. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015;61:361–367. doi: 10.1093/cid/civ308. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources