Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions
- PMID: 31973054
- PMCID: PMC7037355
- DOI: 10.3390/s20030580
Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions
Abstract
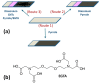
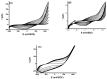
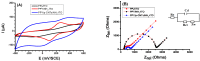
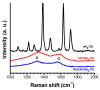
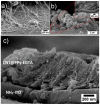
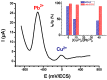
Highly sensitive multicomponent materials designed for the recognition of hazardous compounds request control over interfacial chemistry. The latter is a key parameter in the construction of the sensing (macro) molecular architectures. In this work, multi-walled carbon nanotubes (CNTs) were deposited on diazonium-modified, flexible indium tin oxide (ITO) electrodes prior to the electropolymerization of pyrrole. This three-step process, including diazonium electroreduction, the deposition of CNTs and electropolymerization, provided adhesively-bonded, polypyrrole-wrapped CNT composite coatings on aminophenyl-modified flexible ITO sheets. The aminophenyl (AP) groups were attached to ITO by electroreduction of the in-situ generated aminobenzenediazonium compound in aqueous, acidic medium. For the first time, polypyrrole (PPy) was electrodeposited in the presence of both benzenesulfonic acid (dopant) and ethylene glycol-bis(2-aminoethylether)-tetraacetic acid (EGTA), which acts as a chelator. The flexible electrodes were characterized by XPS, Raman and scanning electron microscopy (SEM), which provided strong supporting evidence for the wrapping of CNTs by the electrodeposited PPy. Indeed, the CNT average diameter increased from 18 ± 2.6 nm to 27 ± 4.8, 35.6 ± 5.9 and 175 ± 20.1 after 1, 5 and 10 of electropolymerization of pyrrole, respectively. The PPy/CNT/NH2-ITO films generated by this strategy exhibit significantly improved stability and higher conductivity compared to a similar PPy coating without any embedded CNTs, as assessed by from electrochemical impedance spectroscopy measurements. The potentiometric response was linear in the 10-8-3 × 10-7 mol L-1 Pb(II) concentration range, and the detection limit was 2.9 × 10-9 mol L-1 at S/N = 3. The EGTA was found to drastically improve selectivity for Pb(II) over Cu(II). To account for this improvement, the density functional theory (DFT) was employed to calculate the EGTA-metal ion interaction energy, which was found to be -374.6 and -116.4 kJ/mol for Pb(II) and Cu(II), respectively, considering solvation effects. This work demonstrates the power of a subtle combination of diazonium coupling agent, CNTs, chelators and conductive polymers to design high-performance electrochemical sensors for environmental applications.
Keywords: chelator; diazonium; electrochemical sensors; heavy metal ions; multiwalled carbon nanotubes; polypyrrole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes.Environ Sci Pollut Res Int. 2018 Jul;25(20):20012-20022. doi: 10.1007/s11356-018-2140-x. Epub 2018 May 9. Environ Sci Pollut Res Int. 2018. PMID: 29744780
-
In situ diazonium-modified flexible ITO-coated PEN substrates for the deposition of adherent silver-polypyrrole nanocomposite films.Langmuir. 2014 Aug 12;30(31):9397-406. doi: 10.1021/la501909r. Epub 2014 Jul 29. Langmuir. 2014. PMID: 25027950
-
Preparation, characterization and anion exchange properties of polypyrrole/carbon nanotube nanocomposites.J Nanosci Nanotechnol. 2006 Feb;6(2):547-53. doi: 10.1166/jnn.2006.122. J Nanosci Nanotechnol. 2006. PMID: 16573059
-
A review of fabrication and applications of carbon nanotube film-based flexible electronics.Nanoscale. 2013 Mar 7;5(5):1727-52. doi: 10.1039/c3nr33560g. Epub 2013 Feb 5. Nanoscale. 2013. PMID: 23381727 Review.
-
Recent advances in hybrids of carbon nanotube network films and nanomaterials for their potential applications as transparent conducting films.Nanoscale. 2011 Apr;3(4):1361-73. doi: 10.1039/c0nr00855a. Epub 2011 Feb 28. Nanoscale. 2011. PMID: 21359350 Review.
Cited by
-
Editorial to the Special Issue SELSA: "Sensors for Environmental and Life Science Applications".Sensors (Basel). 2021 Aug 9;21(16):5353. doi: 10.3390/s21165353. Sensors (Basel). 2021. PMID: 34450795 Free PMC article.
-
Polymeric Nanocomposites for Environmental and Industrial Applications.Int J Mol Sci. 2022 Jan 18;23(3):1023. doi: 10.3390/ijms23031023. Int J Mol Sci. 2022. PMID: 35162946 Free PMC article. Review.
-
The Use of Factorial Design and Simplex Optimization to Improve Analytical Performance of In Situ Film Electrodes.Sensors (Basel). 2020 Jul 14;20(14):3921. doi: 10.3390/s20143921. Sensors (Basel). 2020. PMID: 32674513 Free PMC article.
-
Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection.Sensors (Basel). 2020 Nov 14;20(22):6502. doi: 10.3390/s20226502. Sensors (Basel). 2020. PMID: 33202533 Free PMC article.
-
Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors.Sensors (Basel). 2021 Jun 23;21(13):4300. doi: 10.3390/s21134300. Sensors (Basel). 2021. PMID: 34201852 Free PMC article. Review.
References
-
- Iijima S., Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;364:603–737. doi: 10.1038/363603a0. - DOI
-
- Wang J., Du S., Onodera T., Yatabe R., Tanaka M., Okochi M., Toko K. An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives. Sensors. 2018;18:4461. doi: 10.3390/s18124461. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

