Physicochemical Foundations of Life that Direct Evolution: Chance and Natural Selection are not Evolutionary Driving Forces
- PMID: 31973071
- PMCID: PMC7175370
- DOI: 10.3390/life10020007
Physicochemical Foundations of Life that Direct Evolution: Chance and Natural Selection are not Evolutionary Driving Forces
Abstract
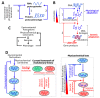
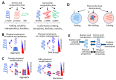
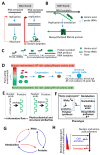
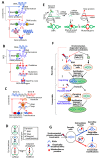
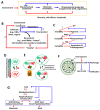
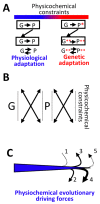
The current framework of evolutionary theory postulates that evolution relies on random mutations generating a diversity of phenotypes on which natural selection acts. This framework was established using a top-down approach as it originated from Darwinism, which is based on observations made of complex multicellular organisms and, then, modified to fit a DNA-centric view. In this article, it is argued that based on a bottom-up approach starting from the physicochemical properties of nucleic and amino acid polymers, we should reject the facts that (i) natural selection plays a dominant role in evolution and (ii) the probability of mutations is independent of the generated phenotype. It is shown that the adaptation of a phenotype to an environment does not correspond to organism fitness, but rather corresponds to maintaining the genome stability and integrity. In a stable environment, the phenotype maintains the stability of its originating genome and both (genome and phenotype) are reproduced identically. In an unstable environment (i.e., corresponding to variations in physicochemical parameters above a physiological range), the phenotype no longer maintains the stability of its originating genome, but instead influences its variations. Indeed, environment- and cellular-dependent physicochemical parameters define the probability of mutations in terms of frequency, nature, and location in a genome. Evolution is non-deterministic because it relies on probabilistic physicochemical rules, and evolution is driven by a bidirectional interplay between genome and phenotype in which the phenotype ensures the stability of its originating genome in a cellular and environmental physicochemical parameter-depending manner.
Keywords: Darwinism; Evolution; Origin of life; RNA; biophysics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Physics-Biology continuum challenges darwinism: Evolution is directed by the homeostasis-dependent bidirectional relation between genome and phenotype.Prog Biophys Mol Biol. 2021 Dec;167:121-139. doi: 10.1016/j.pbiomolbio.2021.05.008. Epub 2021 Jun 4. Prog Biophys Mol Biol. 2021. PMID: 34097984 Review.
-
Phenotypic Evolution With and Beyond Genome Evolution.Curr Top Dev Biol. 2016;119:291-347. doi: 10.1016/bs.ctdb.2016.04.002. Epub 2016 May 11. Curr Top Dev Biol. 2016. PMID: 27282029 Review.
-
Mutations, evolution and the central role of a self-defined fitness function in the initiation and progression of cancer.Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):162-166. doi: 10.1016/j.bbcan.2017.03.005. Epub 2017 Mar 21. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28341421 Free PMC article. Review.
-
Physiology is rocking the foundations of evolutionary biology.Exp Physiol. 2013 Aug;98(8):1235-43. doi: 10.1113/expphysiol.2012.071134. Epub 2013 Apr 12. Exp Physiol. 2013. PMID: 23585325
-
Somatic clonal evolution: A selection-centric perspective.Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):139-150. doi: 10.1016/j.bbcan.2017.01.006. Epub 2017 Feb 2. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28161395 Review.
Cited by
-
Facts, Dogmas, and Unknowns About Mitochondrial Reactive Oxygen Species in Cancer.Antioxidants (Basel). 2024 Dec 19;13(12):1563. doi: 10.3390/antiox13121563. Antioxidants (Basel). 2024. PMID: 39765891 Free PMC article. Review.
-
Origin of Life: The Point of No Return.Life (Basel). 2020 Nov 3;10(11):269. doi: 10.3390/life10110269. Life (Basel). 2020. PMID: 33153087 Free PMC article.
-
The origin of genetic and metabolic systems: Evolutionary structuralinsights.Heliyon. 2023 Mar 11;9(3):e14466. doi: 10.1016/j.heliyon.2023.e14466. eCollection 2023 Mar. Heliyon. 2023. PMID: 36967965 Free PMC article.
-
Non-Random Genome Editing and Natural Cellular Engineering in Cognition-Based Evolution.Cells. 2021 May 7;10(5):1125. doi: 10.3390/cells10051125. Cells. 2021. PMID: 34066959 Free PMC article. Review.
-
Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy.Biomolecules. 2023 Sep 17;13(9):1402. doi: 10.3390/biom13091402. Biomolecules. 2023. PMID: 37759802 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

