Penetrating Traumatic Brain Injury Triggers Dysregulation of Cathepsin B Protein Levels Independent of Cysteine Protease Activity in Brain and Cerebral Spinal Fluid
- PMID: 31973644
- PMCID: PMC7307674
- DOI: 10.1089/neu.2019.6537
Penetrating Traumatic Brain Injury Triggers Dysregulation of Cathepsin B Protein Levels Independent of Cysteine Protease Activity in Brain and Cerebral Spinal Fluid
Abstract
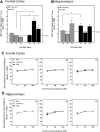
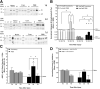
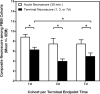
Cathepsin B (CatB), a lysosomal cysteine protease, is important to brain function and may have dual utility as a peripheral biomarker of moderate-severe traumatic brain injury (TBI). The present study determined levels of pro- and mature (mat) CatB protein as well as cysteine protease activity within the frontal cortex (FC; proximal injury site), hippocampus (HC; distal injury site), and cerebral spinal fluid (CSF) collected 1-7 days after craniotomy and penetrating ballistic-like brain injury (PBBI) in rats. Values were compared with naïve controls. Further, the utility of CatB protein as a translational biomarker was determined in CSF derived from patients with severe TBI. Craniotomy increased matCatB levels in the FC and HC, and led to elevation of HC activity at day 7. PBBI caused an even greater elevation in matCatB within the FC and HC within 3-7 days. After PBBI, cysteine protease activity peaked at 3 days in the FC and was elevated at 1 day and 7 days, but not 3 days, in the HC. In rat CSF, proCatB, matCatB, and cysteine protease activity peaked at 3 days after craniotomy and PBBI. Addition of CA-074, a CatB-specific inhibitor, confirmed that protease activity was due to active matCatB in rat brain tissues and CSF at all time-points. In patients, CatB protein was detectable from 6 h through 10 days after TBI. Notably, CatB levels were significantly higher in CSF collected within 3 days after TBI compared with non-TBI controls. Collectively, this work indicates that CatB and its cysteine protease activity may serve as collective molecular signatures of TBI progression that differentially vary within both proximal and distal brain regions. CatB and its protease activity may have utility as a surrogate, translational biomarker of acute-subacute TBI.
Keywords: biomarkers; cathepsin B; clinical TBI; cysteine protease; head trauma; penetrating ballistic-like brain injury; translational rodent models; traumatic brain injury.
Conflict of interest statement
V. Hook, G. Hook, and S. Jacobsen have equity positions and G. Hook and S. Jacobsen are employed by American Life Science Pharmaceuticals, Inc. V. Hook's conflict has been disclosed and is managed by her employer, the University of California, San Diego. The other authors have no conflicts of interest.
Figures



References
-
- Brickell T.A., Lange R.T., and French L.M. (2014). Three-year outcome following moderate-to-severe tbi in US military service members: a descriptive cross-sectional study. Military Med. 179, 839–848 - PubMed
-
- Orman J.A., Geyer D., Jones J., Schneider E.B., Grafman J., Pugh M.J., and DuBose J. (2012). Epidemiology of moderate-to-severe penetrating versus closed traumatic brain injury in the Iraq and Afghanistan wars. J. Trauma Acute Care 73, S496–S502 - PubMed
-
- Schulz-Heik R.J., Poole J.H., Dahdah M.N., Sullivan C., Date E.S., Salerno R.M., Schwab K., and Harris O. (2016). Long-term outcomes after moderate-to-severe traumatic brain injury among military veterans: successes and challenges. Brain Inj. 30, 271–279 - PubMed

