Biogenesis and Functions of Circular RNAs Come into Focus
- PMID: 31973951
- PMCID: PMC7069689
- DOI: 10.1016/j.tcb.2019.12.004
Biogenesis and Functions of Circular RNAs Come into Focus
Abstract
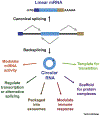
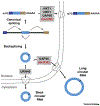
Many eukaryotic protein-coding genes are able to generate exonic circular RNAs. Most of these covalently linked transcripts are expressed at low levels, but some accumulate to higher levels than their associated linear mRNAs. We highlight several methodologies that have been developed in recent years to identify and characterize these transcripts, and which have revealed an increasingly detailed view of how circular RNAs can be generated and function. It is now clear that modulation of circular RNA levels can result in a variety of molecular and physiological phenotypes, including effects on the nervous system, innate immunity, microRNAs, and many disease-relevant pathways.
Keywords: backsplicing; circRNA; innate immunity; microRNA; pre-mRNA splicing; translation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

