Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes
- PMID: 31974163
- PMCID: PMC7062177
- DOI: 10.1074/jbc.REV119.010155
Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes
Abstract
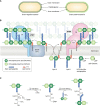
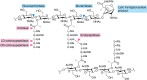
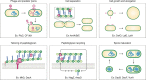
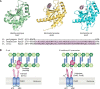
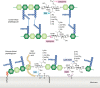
Bacteria account for 1000-fold more biomass than humans. They vary widely in shape and size. The morphological diversity of bacteria is due largely to the different peptidoglycan-based cell wall structures that encase bacterial cells. Although the basic structure of peptidoglycan is highly conserved, consisting of long glycan strands that are cross-linked by short peptide chains, the mature cell wall is chemically diverse. Peptidoglycan hydrolases and cell wall-tailoring enzymes that regulate glycan strand length, the degree of cross-linking, and the addition of other modifications to peptidoglycan are central in determining the final architecture of the bacterial cell wall. Historically, it has been difficult to biochemically characterize these enzymes that act on peptidoglycan because suitable peptidoglycan substrates were inaccessible. In this review, we discuss fundamental aspects of bacterial cell wall synthesis, describe the regulation and diverse biochemical and functional activities of peptidoglycan hydrolases, and highlight recently developed methods to make and label defined peptidoglycan substrates. We also review how access to these substrates has now enabled biochemical studies that deepen our understanding of how bacterial cell wall enzymes cooperate to build a mature cell wall. Such improved understanding is critical to the development of new antibiotics that disrupt cell wall biogenesis, a process essential to the survival of bacteria.
Keywords: Lipid II; LytR-CpsA-Psr ligases; O-acetyltransferase; acetyltransferase; antibiotic development; antibiotics; cell wall biochemistry; hydrolase; peptidoglycan; peptidoglycan hydrolase; peptidoglycan hydrolase regulators; teichoic acid.
© 2020 Do et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

